Final Review

Final Review
The final exam is on Monday April 30, at 12:30 in the regular room. It will consist of two parts, a multiple choice section which will cover the entire semester and the second part, which will be over material we covered since the last exam Look at the suggested problems. Look over previous exams, quizzes, and exam reviews, and the review below.
If you have time, go through old homework and work homework problems from the new material.
Review
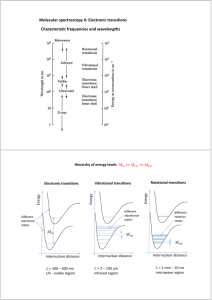
We talked about light interacting with matter in the context of Absorption, Stimulated
Emission, and Spontaneous emission, and talked about how the width of the peak in the spectrum was related to the lifetime, essentially by the Heisenberg uncertainty principle,
E
> ħ/2 the longer the lifetime the narrower the bandwidth. The rate of emission was proportional to the excitation frequency to the third power (Einstein’s A, the spontaneous emission coefficient). Thus an absorption due to excitation in the visible region of the spectrum has a higher frequency associated with the transition (
E = h
), a shorter lifetime, and a broader bandwidth than a peak in the microwave part of the spectrum.
This is based upon the natural lifetime. Remember that another contribution to the bandwidth is collisional deactivation and the bandwidth in solution is broad compared to a gas phase spectrum where you can see vibrational substructure.
In order to be quantitative we use the Beer-Lambert Law. You should know the terms transmission, absorbance, and the relationship to the concentration, the molar absorptivity, or extinction coefficient. What is necessary if one wishes to determine the concentrations of the absorbing species in a two component solution. What is an isobestic point. If there are two or more isobestics in the spectra of a series of solutions of the same total concentration, how many components are in that spectra region.
You should know some general absorption characteristics like if a substance is colorless, does it absorb in the visible part of the spectrum. If it is a particular color, what light wavelengths are absorbed? Which amino acids absorb in the UV region around 260, 230,
200nm? Is there a difference in the UV absorbance of polypeptides vs the amino acids.
Does the secondary structure affect the absorption? Why? What about for the nucleosides, nucleotides, and polynucleotides? What are hypochromicity and hyperchromicity. What is a chromophore?
Fluorescence occurs naturally in some biological molecules. It can be used to determine if a particular molecule is present and give quantitative information. If a particular biological molecule of interest does not fluoresce, fluorescent tags can be covalently bonded or physically adsorbed to make the molecule fluorescent. The lifetime of the excited state as measured by its fluorescence is modeled as first order decay. The mechanisms for deexcitation include a rate constant for fluorescence, thermal deactivation, photochemistry, and quenching. The observed lifetime is then:
= 1/(k f
+ k t
+ k p
+ k
Q
[Q]) and the quantum yield of the fluorescence,
f
, is defined as the number of photons fluoresced divided by the number of photons absorbed. The rate constant k f
is just the
Einstein coefficient of spontaneous emission and k f
= 1/
0
. Thus
f
=
/
0
. Oxygen is a quenching molecule in many situations.
Another type of interaction of light with matter is scattering. We talked about Rayleigh
Scattering, Raman Scattering (both Stokes and anti-Stokes Raman Scattering) and we also talked about the selection rule that the polarizability of the molecule must change as a result of the vibration for the vibrational mode to be Raman active. What is the selection Rule for an IR active vibrational mode? Both IR and Raman Spectroscopy probe the vibrational modes of the molecule. How many vibrational modes are there for a given molecule? It depends on if they are linear or nonlinear, why?
What is anharmonicity? What are hot bands and overtones? Where in the IR spectrum do we expect them to be. What is rotational-vibrational structure. What are the P, Q, and
R branches? In rotation what is the centrifugal distortion constant?
What are Boltzmann statistics. How is the population of one energy related to the population of another energy level. How are these related to the temperature.
The birefringence in a molecule and chirality lead to optical rotation and circular dichroism being useful as a probe for molecules with chiral structures. Thus molecules can be characterized by polarized light. You should be able to explain how these techniques can be used and what the difference is between the two. Is the circular dichroism spectrum the same for the nucleoside or amino acid as it is for the polynucleotide or the protein? What structural affects contribute.
The last topic is NMR which stands for ? This spectroscopy monitors changes in the nuclear spin state. Nuclei can have both integral and half integral spin states. In the presence of a magnetic field the spin states of a nucleus are no longer degenerate and the energy separation between the states depends on the strength of the field and the gyromagnetic ratio of the nucleus. That is
E =
h/2
B
0
= h
L
where
L
is the frequency of the transition in the imposed magnetic field and is called the Larmor frequency. The
Larmor frequency is 500MHz for a proton in a field B o
= 11.7 T. Given a table of
, you should be able to give the Larmor frequency for other spin ½ nuclei as well. What are some of the common spin ½ nuclei. If an NMR instrument is a 300MHz instrument, what would the value of its magnetic field strength be. Why is NMR not considered to be a very sensitive technique in the sense that it can measure concentration only at the % level?
In NMR two important features that alter the transition frequency band are chemical splitting and spin spin coupling. You should be able to explain each of these? Also given a spectrum and a molecule you should be able to identify which peaks are associated with a given NMR band. What is the spin-lattice relaxation time constant T1 and what is the other relaxation constant that is related to the bandwidth of the NMR peaks. For the two dimensional NMR techniques, what is the difference between COSY and NOESY. What is the Nuclear Overhauser Effect. What is it dependence on distance.
About how far away will neighbors make an effect?