Protocol Submission Form - Sistema Universitario Ana G. Méndez
advertisement

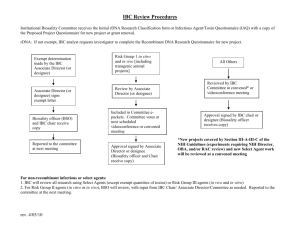
Ana G. Méndez University System Vice Presidency for Planning and Academic Affairs Associate Vice Presidency for Sponsored Programs and Compliance Office of Regulatory Compliance Institutional Biosafety Committee (IBC) PROTOCOL/RESEARCH SUBMISSION FORM New registration I. Resubmitted registration IBC Number: Principal Investigator Basic Information 1. Complete Name: 2. E-mail: 3. Phone/ Fax: 4. Department/School: 5. AGMUS Institution: 6. Category: II. Additional Information Regular Faculty Visiting Faculty Graduate Student Other: Mentor Co-Investigator 1. Complete Name: 2. E-mail: 3. Phone/ Fax: 4. Department/School: 5. Institution: 6. Category: Regular Faculty Visiting Faculty Graduate Student Other: III. Additional Personnel Identify the personnel involved in the project (including students, technicians, temporary help, etc.) and their respective responsibilities. Provide a copy of their curriculum vitae indicating their level of training and experience in working with biological agents. P.O. Box 21345 San Juan, PR 00928-1345 Phone: 787-751-0178 ext. 7195, 7197 FAX: 787-759-6411 compliance.suagm.edu AGMUS-IBC01 April-2012 1 Name: E-mail: Phone/ Fax: Department/School: AGMUS Institution: Project Responsibility: Name: E-mail: Phone/ Fax: Department/School: AGMUS Institution: Project Responsibility: *If more space is needed please attach the additional personnel to this form, using the format provided above. IV. Basic Protocol/ Research Information 1. Project Title: 2. Estimated Project duration: 3. Type of funding: Funding Agency (if applicable): Institutional Non-funded External, Specify: V. Laboratory Basic Information 1. Registered Laboratory Yes No* *If No, IBC_05 form must be completed and submitted 2. Laboratory Location (Institution, campus, building, room, etc.) 3. Identify Risk Group of the biological agent/ material or rDNA/ rRNA 4. Identify the Laboratory Biosafety Level a. Involving Plants (Appendix P of NIH Guidelines) IBC_01-Submission Form Revised (4/2012) RG1 RG2 RG3 BSL-1 BSL-2 N/A* N/A *Research or academic laboratories where no biological agents or rDNA/rRNA are currently stored or used. BSL1-P BSL2-P N/A AGMUS Eng_Ver_01 2 b. Involving Animals (Appendix Q of NIH Guidelines) 5. Identify the existence of a Biological Safety Cabinet in the laboratory (if applicable): BSL1-N BSL2-N N/A Yes No N/A a. Biological Safety Cabinet (BSC) Type Class I Class II-A Class II- B1 Class II-B2 Class II-B3 Class III b. Is it certified? Yes No Yes No N/A Yes No N/A Yes No N/A 6. Identify the availability of a fume hood in the laboratory (if applicable): a. Is it certified? b. Are there available storage cabinets (if applicable)? a. The storage cabinet is recommended for: Flammable Corrosive N/A Other, specify: VI. Project/ Protocol/ Grant Information Include a copy of the Project/ Protocol/ Grant proposal with this registration as an attachment. Include all necessary documents and registrations required by IBC prior to initiation of the project. Biological agents, rDNA/ rRNA and hazardous chemicals to be used shall be registered with the IBC. Be sure to include the following points in your research protocol: Research Study Proposal Study rationale or description Specific aims Detailed Experiment Description (The IBC will evaluate the risks of biological agents and chemical substances to be used) Experimental design Potential biohazards, precautions to be taken and waste management (biological and chemical)- IBC Form 9, Risk Assessment Form VII. Biological Agents Registration Yes No N/A Complete this part if you intend to use and/or store infectious biological agents that presents a risk or potential risk to the health of humans and/or animals. Refer to the IBC Policy and Procedures in order to make a risk assessment of the biohazardous IBC_01-Submission Form Revised (4/2012) AGMUS Eng_Ver_01 3 materials based on the Risk Group (RG) of the agent (RG-1, RG-3). The Biosafety in Microbiological and Biomedical Laboratories (BMBL), which is published by the U.S. Department of Health and Human Services’ Centers for Disease Control and Prevention and the National Institutes of Health, provides recommended biosafety standards for specific organisms. 1. Proposed Research a. Provide a summary of your planned use for the biological agent (s), including significant risk, if any. Note: you must attach (include) a copy of your protocol. b. Provide a written emergency plan for handling accidental spills and personnel exposure (if applicable). c. Will ionizing radiation be used with this agent? Yes No d. Will live animals be infected with this agent? *If, yes, you must obtain prior approval from the Animal Care and Use Committee. *Yes No Remember to include all biological agents in the IBC Form 9- Risk Assessment. VIII. Recombinant DNA/RNA Registration Yes No N/A All activity involving the use and/or storage of rDNA/rRNA must be registered with and approved by the IBC. The NIH Guidelines for Research Involving Recombinant DNA can be found at: http://oba.od.nih.gov/rdna/nih_guidelines_oba.html. Biosafety Assessment RG1 RG2 RG3 a. Identify Risk Group of the rDNA/ rRNA RG-1 – Agents that are not associated with disease in healthy adult humans. RG-2 – Agents that are associated with human disease which is rarely serious and for which preventive or therapeutic interventions are often available. RG-3 – Agents that are associated with serious or lethal human disease for which preventive or therapeutic interventions may be available (high individual risk but low community risk). b. Identify the NIH Guidelines category of rDNA/rRNA research (check all that apply) Section III-A (require IBC and NIH director approval before initiation) Section III-D (require only IBC approval before initiation) IBC_01-Submission Form Revised (4/2012) Section III-B (require NIH/OBA and IBC approval before initiation) Section III-E (require IBC notice simultaneously with initiation) Section III-C (require IBC and IRB approval before initiation) Section III-F (exempt experiments) AGMUS Eng_Ver_01 4 Proposed Research with rDNA/ rRNA a. Provide a summary of your planned use of rDNA/ rRNA, including significant risk, if any. If you intend this registration to include multiple constructs, please list them. Note: you must attach (include) a copy of your protocol. b. Provide a written emergency plan for handling accidental spills and personnel exposure to rDNA/rRNA (if applicable). Additional information a. Section III-A, III-B and III-C Research Only: Attach a copy of all relevant information submitted to NIH or NIH/OBA. b. Section III-D, III-E and/or III-F Research Only: Provide the following information: i. Sources of rDNA/rRNA ii. The nature of the inserted DNA sequences iii. Do you plan to propagate the recombinant? Yes No *Yes No Yes No *Yes No iv. What is the host recipient(s)/ vector(s)/ specific phage or plasmid? v. Is a helper virus to be used? *If yes, what is the helper virus vi. Are plants or animals to be exposed to the rDNA/rRNA? vii. Will an attempt be made to obtain expression of a foreign gene? *If yes, what protein(s) will be produced? c. Describe the containment conditions to be implemented IBC_01-Submission Form Revised (4/2012) AGMUS Eng_Ver_01 5 IX. Hazardous Chemicals Report All chemicals or mixture of elements and/or compounds which represent a physical or health hazard to humans under the registered investigation must be informed to the IBC. Use this form to report any chemical which falls within the following categories: flammable, combustible, explosive, oxidizing, pyrophoric, reactive, organic peroxide or compressed gas. Note that chemicals are not subject to registration, but they do have to be reported to the IBC as they may pose a threat to personnel if handled without caution. Proposed Research a. Provide a summary of your planned use for the all chemicals or mixture of elements and/or compounds, including significant risk, if any. Include descriptions of the procedures, concentration at which it will be used, the frequency of use, etc. Note: you must attach (include) a copy of your protocol b. Provide a written emergency plan for handling accidental spills and personnel exposure (if applicable). Remember to include all chemicals reactants to be used in the IBC Form 9- Risk Assessment. X. Applicant’s Agreement The signature certifies that the PI understands and accepts the following obligations in this study: I recognize that as the PI it is my responsibility to ensure that this research will conform with the IBC approved protocol and the provisions of the NIH Guidelines for Research Involving Recombinant DNA, the CDC/NIH Biosafety in Microbiological and Biomedical Laboratories manual, and the Select Agent Rule (http://www.cdc.gov/od/ohs/Irsat/42cfr72.htm) where appropriate. I will oversee the development and implementation of standard operating procedures to secure the Biological Safety in the laboratory. I will inform the IBC of any unanticipated biosafety related problems encountered while doing the research. Any significant proposed changes, including addition of new personnel, will be reported to and approved by the IBC before the change is implemented or personnel added. I will maintain all required laboratory management records on file and I recognize that representatives of the Office of Regulatory Compliance are authorized to inspect these records. I understand that it is my responsibility to assure that all associated personnel are trained in the laboratory safety practices required for the work described. IBC_01-Submission Form Revised (4/2012) AGMUS Eng_Ver_01 6 I understand that failure to comply with NIH regulations, IBC requirements/policies, and the provisions of the protocol as approved by the IBC may result in suspension or termination of my research project. I understand that IBC approval is valid for up to three years, subjected to a yearly submission of a continuation form (IBC Form 7). Any spill of biological agents, chemical hazards or rDNA/rRNA, any equipment or facility failure (e.g., ventilation failure), and/or any breakdown in procedure that could result in potential exposure of laboratory personnel and/or the public to infectious material will be reported to the Office of Regulatory Compliance. I understand that if I use the project described above as a basis for a funding proposal (either intramural or extramural), it is my responsibility to ensure that the description of the work in it is identical in principle to the one contained in this registration. The information provided herein is accurate to the best of my knowledge. / / (mm/dd/yy) / Signature of Principal Investigator / / (mm/dd/yy) / Signature of Dean Submit this completed form to the AGMUS Office of Regulatory Compliance. IBC Use Only Exempt Non-Exempt Approved Identify Biosafety level approved: Disapproved BSL-1 BSL-2 Laboratory inspection required prior to initiation of use? N/A Yes* No *Use of infectious agents cannot begin until the IBC or its designee has inspected the laboratory and approved it for BSL-2. IBC Chair Signature / / (mm/dd/yy) / IBC-signed copy returned to Registrant IBC_01-Submission Form Revised (4/2012) AGMUS Eng_Ver_01 7