IBC Review Procedures
advertisement
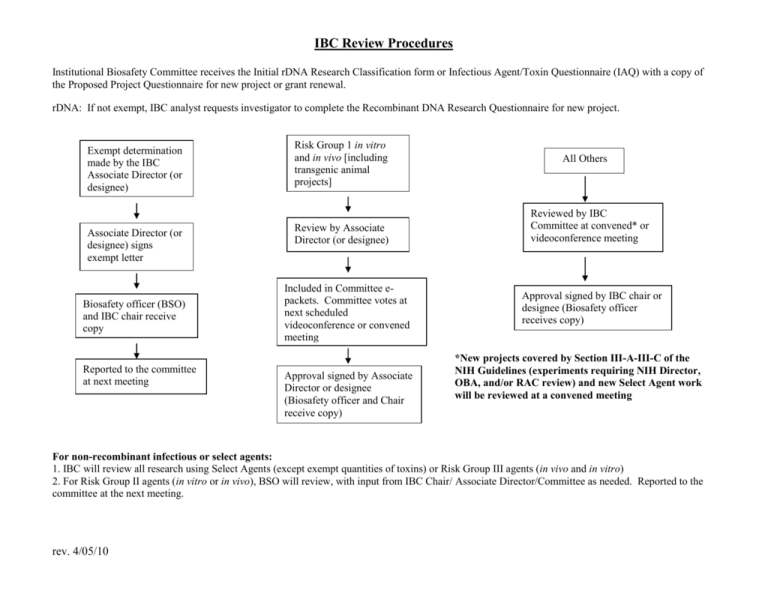
IBC Review Procedures Institutional Biosafety Committee receives the Initial rDNA Research Classification form or Infectious Agent/Toxin Questionnaire (IAQ) with a copy of the Proposed Project Questionnaire for new project or grant renewal. rDNA: If not exempt, IBC analyst requests investigator to complete the Recombinant DNA Research Questionnaire for new project. Exempt determination made by the IBC Associate Director (or designee) Associate Director (or designee) signs exempt letter Biosafety officer (BSO) and IBC chair receive copy Reported to the committee at next meeting Risk Group 1 in vitro and in vivo [including transgenic animal projects] Review by Associate Director (or designee) Included in Committee epackets. Committee votes at next scheduled videoconference or convened meeting Approval signed by Associate Director or designee (Biosafety officer and Chair receive copy) All Others Reviewed by IBC Committee at convened* or videoconference meeting Approval signed by IBC chair or designee (Biosafety officer receives copy) *New projects covered by Section III-A-III-C of the NIH Guidelines (experiments requiring NIH Director, OBA, and/or RAC review) and new Select Agent work will be reviewed at a convened meeting For non-recombinant infectious or select agents: 1. IBC will review all research using Select Agents (except exempt quantities of toxins) or Risk Group III agents (in vivo and in vitro) 2. For Risk Group II agents (in vitro or in vivo), BSO will review, with input from IBC Chair/ Associate Director/Committee as needed. Reported to the committee at the next meeting. rev. 4/05/10











