Testing and Issuance
advertisement

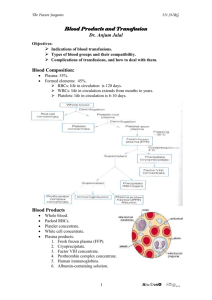
MedTraining: Transfusion Safety: Testing and Issuance 51 Points NAME______________________________ 1. When transfusing what must be considered for: (1.5 point) a. Whole blood transfusions b. Red cells c. plasma 2. What percentage of the population is O negative? (0.5 point) 3. List 4 conditions for which red blood cells (RBCs) are transfused.(2 points) 4. For what condition are white blood cells transfused for? (1 point) 5. List 5 conditions for which platelets are transfused. (2.5 points) 6. List 6 conditions for which fresh frozen plasma is transfused. (3 points) 7. When is FFP thawed? Why? (1 points) 8. List 3 conditions for which cryoprecipitate is transfused. (1.5 points) 9. What is the expiration time of pooled cryoprecipitate? (0.5 point) 10. List 6 conditions for which fresh irradiated blood is transfused. (3 points) 11. List 2 blood products which DO NOT require irradiation. (1 point) 12. State the reason that the products listed in question11 DO NOT require irradiation. (0.5 point) 13. What is the purpose for leukoreducing red blood cells? (1 point) 14. What is meant by “Zero Tolerance” as it relates to blood bank specimens? (1 point) 15. List 4 items which would cause rejection of a blood bank sample. (2 points) 16. After completing sample identification what is the next step in the process of blood bank testing? (1 point) 17. List 3 components which DO NOT require a Type and Crossmatch. (1.5 points) 18. State why crossmatched and uncrossmatched blood are stored separately. (1 point) 19. Why are compatibility results valid for only 3 days? (1 point) 20. For urgent requests what form must a physician complete before issuing blood before the crossmatch is completed? (0.5 points) 21. List 4 pieces of information which must be verified on the “caution tag”? (2 points) 22. State within what time frame a transfusion must be started AND completed. (1 point) 23. How many units can be released for a patient at one time? (0.5 points) 24. List the 7 pieces of information which must be confirmed by the courier and laboratory staff member prior to issuing a component for transfusion. (3.5 points) 25. List 3 items which should be inspected prior to release of blood for transfusion. (1.5 points) 26. For fresh frozen plasma state the additional label that must be affixed to the product. (0.5 point) 27. Prior to release for transfusion list 4 items which must be confirmed or observed. (2 points) 28. Describe observations for abnormal appearance for: (4 points) a. Red blood cells b. Platelets c. Thawed plasma d. Cryoprecipitate 29. When observing the plasma in a sprig what would the following indicate? (4 point) a. Icterus b. Lipemia c. Clots in red blood cells d. Purplish color or blackish masses in red blood cells 30. Upon completion of patient identification information and component inspection what must 3 items must be documented? (1.5 points) 1 31. What 5 items must be documented when a blood product is returned due to a suspected transfusion reaction? (2.5 points) 32. Within what time period must a FATAL transfusion reaction be reported? (0.5 point) 33. What organization is the fatal transfusion reaction reported to? (0.5 points) 2