MediKredit Integrated Healthcare Solutions (Pty) Ltd
advertisement

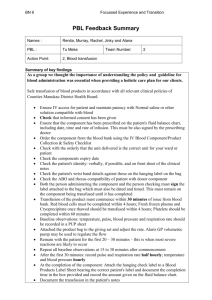
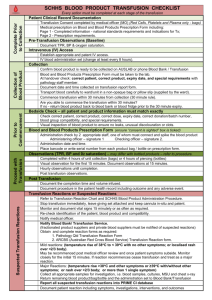
NAPPI Allocation Rules – Biological Products Manufacturer requirements: The applicant must be a legal entity and the product must be produced by a Blood Transfusion Service which is: o Licensed by the Department of Health for the purposes of section 19 of the Human Tissue Act (No. 65 of 1983) in terms of regulation 3 of the Regulations Relating to Blood and Blood Products (No. R.1935 of 17 August 1990). o Fully accredited by South African National Accreditation Services (SANAS), or equivalent body, in terms of the SANAS Requirements for Accreditation of Blood Transfusion Services and ISO/IEC 17025 interpreted for Medical Laboratories. Product requirements: Biological products are not required to be registered by the Medicines Control Council and therefore do not have registration numbers etc. However, for blood products to be allocated a NAPPI the product must be produced in accordance with the Standards for Practice of Blood Transfusion in South Africa published by assent of the Minister of Health in terms of section 37 of the Human Tissues Act (No. 65 of 1983) and the Regulations Relating to Blood and Blood Products (No. R. 1935 of 17 August 1990). For Biological Products Templates please visit our Website: http://www.medikredit.co.za