Photosynthesis Worksheet
First Impressions of the Halogens
This worksheet is designed to be completed alongside the lesson.
The icons on the left tell you which pages of the lesson the questions relate to.
Name: Date:
In this lesson you are going to investigate the elements in Group 7 of the Periodic
Table. Group 7 elements are also known as the halogens and they all have similar properties.
1
2
3
1. List all the halogens and their symbols as they appear in the Periodic
Table from top to bottom.
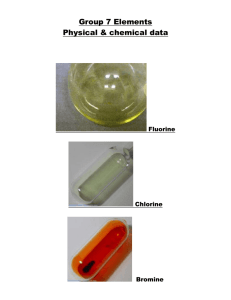
2. a. Describe the appearance of chlorine, bromine and iodine. b. Do the halogens get lighter or darker as you move down the group? c. Do the melting points of these three halogens increase or decrease as you go down the group? What evidence from the images tells you this?
3. a. Fill in the table with the melting points of chlorine, bromine and iodine.
Chlorine Bromine Iodine
Melting point ( C)
Version 2.0
Copyright © 2005 PLATO Learning (UK) Ltd. All rights reserved.
1
First Impressions of the Halogens b. Plot the melting points for chlorine, bromine and iodine on the graph below and connect the points.
300
-
200
( C)
100
0
–100
F Cl Br I At
–200 c. Extend your line on the graph to fluorine and astatine and estimate their melting points.
Fluorine Astatine
Estimated melting point ( C)
4. a. Now fill in the table with the boiling points of chlorine, bromine and iodine. Then plot the boiling points on the graph below.
Chlorine Bromine Iodine
Boiling point ( C)
Version 2.0
Copyright © 2005 PLATO Learning (UK) Ltd. All rights reserved.
2
First Impressions of the Halogens b. Plot the boiling points for chlorine, bromine and iodine on the graph below and connect the points.
300
-
200
( C)
100
0
F Cl Br I At
–100
–200 c. Extend your line on the graph to fluorine and astatine and estimate their boiling points.
Fluorine Astatine
Estimated boiling point ( C)
4
5. a. Write down the actual values for the melting points and boiling points of fluorine and astatine.
Fluorine Astatine
Actual melting point ( C)
Actual boiling point ( C)
Version 2.0
Copyright © 2005 PLATO Learning (UK) Ltd. All rights reserved.
3
First Impressions of the Halogens
5 b. c.
How close were your predictions?
Summarise the trends in melting point and boiling point as you go down the group.
6. a. Suggest why the halogen elements are not often used on their own but are combined with other elements as compounds. b. List ten uses of the halogens or their compounds.
Version 2.0
Copyright © 2005 PLATO Learning (UK) Ltd. All rights reserved.
4






![[1] - Boswellsgmt](http://s3.studylib.net/store/data/006603407_1-fadfbce8d94050a9fb3c38a07d86e8ee-300x300.png)