TheElectronCloud
advertisement
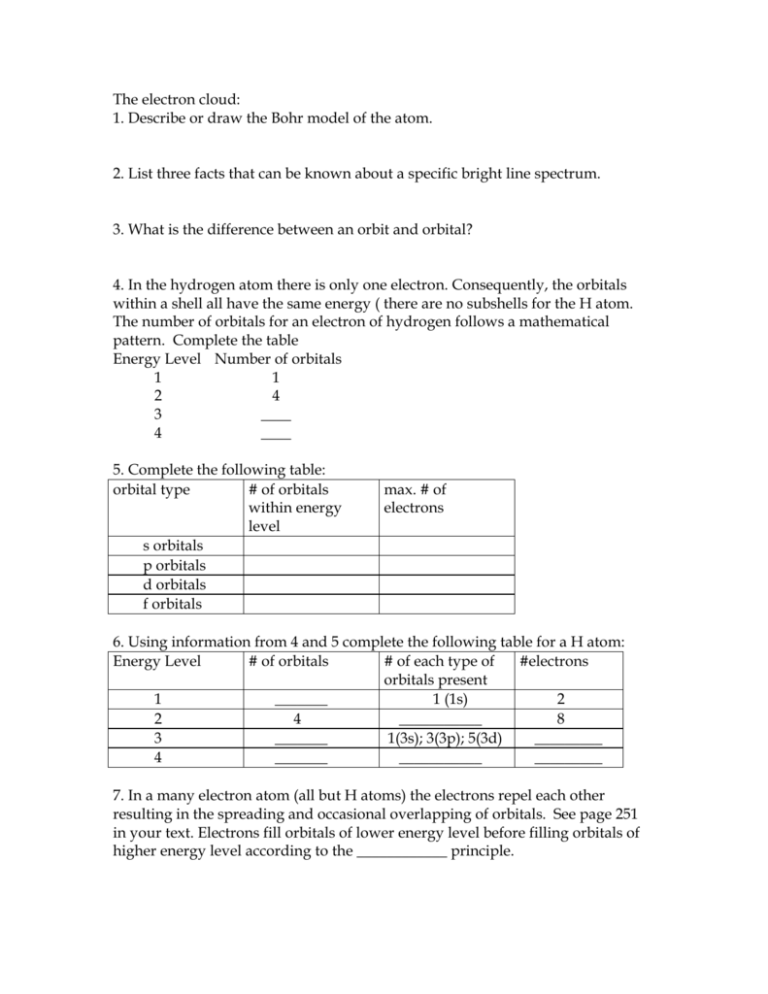
The electron cloud: 1. Describe or draw the Bohr model of the atom. 2. List three facts that can be known about a specific bright line spectrum. 3. What is the difference between an orbit and orbital? 4. In the hydrogen atom there is only one electron. Consequently, the orbitals within a shell all have the same energy ( there are no subshells for the H atom. The number of orbitals for an electron of hydrogen follows a mathematical pattern. Complete the table Energy Level Number of orbitals 1 1 2 4 3 ____ 4 ____ 5. Complete the following table: orbital type # of orbitals within energy level s orbitals p orbitals d orbitals f orbitals max. # of electrons 6. Using information from 4 and 5 complete the following table for a H atom: Energy Level # of orbitals # of each type of #electrons orbitals present 1 _______ 1 (1s) 2 2 4 ___________ 8 3 _______ 1(3s); 3(3p); 5(3d) _________ 4 _______ ___________ _________ 7. In a many electron atom (all but H atoms) the electrons repel each other resulting in the spreading and occasional overlapping of orbitals. See page 251 in your text. Electrons fill orbitals of lower energy level before filling orbitals of higher energy level according to the ____________ principle. 8. Since orbitals overlap, scientists term a collection of orbitals of close energy a shell. Within a shell orbitals such as the 3s may have slightly lower energy level than the 3p orbitals. Thus the 3s and 3p orbitals belong to different __________. 9. Answer questions in the box below. Subshells have a different level of energy. 3p 3d 3d 3p 3d 3p 3d 3d The fourth shell 4s This is a view of the 4th shell. •How many subshells are present? •How many electrons could be kept in the 4th shell? •How many elements in the 4th row of the periodic table? 10. Answer questions in the box below. Draw in lines to show how orbitals fills according to the aufbau principle. 1s 2s 3s 4s 5s 6s 7s 2p 3p 3d 4d 4f 5f 4p 5p 5d 6d 6p ? •How does this diagram relate to the elements in each row of the periodic table? •Each row begins with an 's' orbital and except for the first row, each row ends with a ________orbital. •Why does a ? replace 7p in the above diagram? •How many elements are in the 6th row (6s to 6p)? 11. What is Pauli's Exclusion principle? 12. Write the complete electron configuration that would describe: A) 7 electrons B) 15 electrons C) 33 electrons D) 51 electrons 12. Identify each element in question 11 A) B) C) D) 13. Write the electron configuration for each element described in question 12, using noble gas cores. A) B) C) D) 14. In the electron configuration: 1s22s22p4; A) How many subshells are involved? B) How many orbitals are involved? C) How many electrons are involved? 15. Describe or state Hund's rule. 16. Apply Hund's rule by writing the orbital diagram for an oxygen atom. 17. Write the electron configuration for F, Cl, Br, and I. Use noble gas cores. A) F = B) Cl = C) Br = D) I = 18. Nitrogen in the VA column and 2nd row ends in 2p3. (1s22s22p3). How the do the following elements end? A) 12Mg B) 26Fe C) 19K D) 16S E) 56 Ba F) 30Zn G) 37Rb H) 34Se 19. Any element whose electron configuration ends filling or having filled an 's' orbital is an s-block element. This is similar for any element whose electron configuration is filling or has filled a 'p' (p-block) a 'd' (d-block) or an 'f' orbital is an f-block element. Identify each element in question 18 A-H as being an s-block, p-block, d-block or f-block element. A) B) C) D) E) F) G) H) 20. For the following elements, determine how many electrons are found beyond the noble gas core. For example S = [Ne]3s23p4 would have 6 electrons beyond noble gas core. A) Ca B) Al C) Ga D) O E) As F) Br 21. Valence electrons are the highest s and p electrons beyond the noble gas core. How many valence electrons are present for each element in problem 20. A) Ca B) Al C) Ga D) O E) As F) Br 22. For S = [Ne]3s23p4, the valence electron configuration would be 3s23p4. What is the valence electron configuration for each element in question 20. A) Ca B) Al C) Ga D) O E) As F) Br 23. Is there a relationship between the number of valence electrons for an element and the A family (column on periodic table) to which to element belongs?







