Building Molecules
advertisement

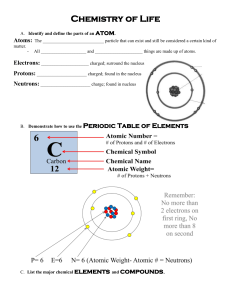
Lab # ______: Building Molecules Date ________ In this exercise you will be using “ball and stick” kits to make models of various molecules. You will use wooden balls drilled with holes to represent atoms and sticks or springs to represent chemical bonds. A chemical bond is energy that can hold 2 atoms together. The molecules you will build in this exercise are held together by covalent bonds. This means that the atoms are held together because they are sharing electrons. In biology we are concerned primarily with four kinds of atoms: 1. Carbon (represented by a black ball) can bond to 4 other atoms. Choose a black ball and insert 4 sticks into its holes (Note that he black ball has only 4 holes) 2. Nitrogen (represented by a blue ball) usually bonds to 3 other atoms in biological molecules. . Choose a blue ball and insert sticks into 3 of the holes in the ball. 3. Oxygen (represented by a red ball) usually bonds to 2 other atoms in biological molecules. Choose a red ball and insert 2 sticks into it. (Note that the red balls have only 2 holes) 4. Hydrogen (represented by a yellow) can bond to 1 other atom. Choose a yellow ball and insert a stick into it. (Note that the yellow balls have only 1 hole) When atoms are held together by chemical bonds they make molecules (for example, the same stick that comes from a carbon also goes into a hydrogen, thus holding the two atoms together). If a molecule is made of only one type of atom, it is called an element. If a molecule is made up of two or more different kinds of atoms it is called a compound. Use balls and sticks to build the following molecules. Remember, you have 4 sticks (bonds) coming out of a carbon atom, 3 sticks (bonds) coming from a nitrogen atom, 2 sticks (bonds) coming from an oxygen atom, and 1 stick (bond) coming from a hydrogen atom. Draw a structural formula for each after you build it. MOLECULAR FORMULA NH3 (ammonia) H2O (water) CH4 (methane) H2 (hydrogen) CH4O (methyl alcohol) STRUCTURAL FORMULA ELEMENT OR COMPOUND? Sometimes atoms form 2 bonds to the same atom. This is called a double bond. To represent a double bond you will need to use something more flexible. You will use the springs included in the kits for these double bonds. Triple bonds (3 bonds between the same two atoms) are done the same way. Using springs to represent double and triple bonds, build these molecules: MOLECULAR FORMULA O2 (oxygen) CO2 (carbon dioxide) C2H4 (ethene) N2 (nitrogen) C2H4O2 (acetic acid – the acid found in vinegar) HINT: Acetic acid is often written as CH3COOH STRUCTURAL FORMULA ELEMENT OR COMPOUND? Sometimes molecules rearrange their chemical bonds with another molecule to form different molecules. For example, O2 and combine with 2 H2 molecules to form 2 H2O molecules: 2 H2 + O2 REACTANTS 2 H2O PRODUCTS The molecules on the left (the ones you start with) are called reactants. The molecules on the right (the ones that are made) are called the products. The change from reactants to products is called a chemical reaction. Build the reactants below. Once you have built them try to rearrange the bonds to make the products shown. Remember that you can only use atoms from the reactants to form the products. REACTANTS #1: #2: N2 + H2 (as many of each as you need to build the products) CO2 + H2 (as many of each as you need to build the products) PRODUCTS NH3 CH4 + O2 A. Write a balanced equation for each chemical reaction. Label the reactants and the products in each. #1: #2: B. Explain the difference between H2 and 2H.