Review for Bonding Test (Jan 2015) – Remember to read Ch. 7 + 8
advertisement

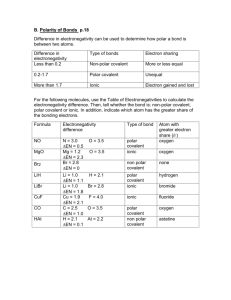
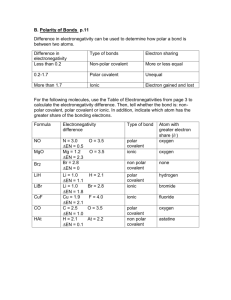
Review for Bonding Test (Jan 2015) – Remember to read Ch. 7 + 8 some on hydrogen bonding (ch12) to review (and see pictures of) these concepts. Especially chapter 7 section 2 (pages 210-217), chapter 8 section 1 (pages 240-245), chapter 8 section 3 (pages 253260), chapter 8 section 4 (pages 261-264), and chapter 12 section 2 (pages 411-414, especially page 413). (Review chapter 10 section 4 (pages 341-350). For empirical and molecular formulas) Know the following concepts VERY WELL: Ionic vs covalent Polar vs Nonpolar Electronegativity Lewis Dot Structures and VSEPR shapes Bonding Given two molecules CH2Cl2 and CH4, how can one be nonpolar and the other polar? Which is nonpolar? Bonding orbitals used & shape: Match with its shape: Nomeclature – name the molecule: Lewis Dot Model: s shape: ___Line_____ H2 ___________________ ________ s2 shape: _linear_______ H2O ___________________ ________ sp2 shape: ________ CH4 ___________________ ________ sp3 shape: ________ BeF2 ___________________ ________ p3 shape: ________ BH3 ___________________ ________ p2 shape: ________ PH3 ___________________ ________ p shape: __Line______ Cl2 ___________________ ________ 1. 2. 3. 4. A covalent bond is created when _______(#) electrons are shared. A triple covalent bonds has _______(#) electrons shared. Ammonia (NH3) makes a_____________shape and is ___________(polar or non-polar). Oxygen has ________(#) pairs of unbonding electrons in a water molecule, these electrons are called ________ pairs. Be able to Draw Lewis Dot Structures for Ionic Compounds, Molecular Compounds and polyatomic ions Draw Lewis Dot Structures, give their VSEPR shapes and determine if the molecules are polar. BeCl2 NI3 ClO3- HClO3 Be able to solve empirical and molecular formula problems 5. A compound with the following composition has a molar mass of 60.10g/mol: 39.97% carbon; 13.41% hydrogen; 46.62% nitrogen. First find the empirical formula then find the molecular formula. 6. Naphthalene is a carbon and hydrogen containing compound often used in moth balls. The empirical formula is C5H4 and its molar mass is 128.16g/mol. Find the molecular formula. 7. Water only has a mass of 18 g/mol and is a liquid at room temperature; N 2 and O2 have a greater molar mass (28 g/ mol and 32 g/ mol). Why is water a liquid and the others gases ? Vocabulary – know definitions and be able to use in the context of a question: Covalent Bond – Ionic Bond – Electron Dot Structures – Octet Rule – Electronegativity – VSEPR Selected Answers: 1. Two 2. Six 3. Pyramidal, polar 4. Two, lone pairs 5. C2H8N2 6. C10H8 Review for Bonding Test – SAMPLE ANSWERS Assigned readings: p.240-243, p.253-257, p. 261-270 Chapter 210-215 Know the following concepts VERY WELL: Why and how atoms ionize To complete an octet or to have the electron configuration of a Nobel gas Ionic vs covalent Ionic give and take to for ions (metal positive cations, negative non-metal anions, covalent SHARE to complete an octet Polar vs Nonpolar Polar means lopsided shape (or pointable); Non-polar are balanced or non-lopsided Water is polar oils are non-polar Electronegativity if the electronegativity on one element is a lot more than the other the compound forms ions (or is “polared apart”) Given two molecules CH2Cl2 and CH4, how can one be nonpolar and the other polar? Which is nonpolar? CH2Cl2 is polar, the chlorine attracts the electrons in bonds better than the hydrogen; CH4 is not lopsided (nonpolar) the hydrogen are distributed evenly around the carbon center. Bonding orbitals used & shape: Match with its shape: Nomeclature – name the molecule: Electron Dot Model: s shape: ___Line_____ H2 s2 shape: _linear_______ H2O _water or dihydrogen monoxide_ ________ sp2 shape: trigonal planer_ CH4 __methane or carbon tetra hydride)_ ________ sp3 shape: _tetrahedral___ BeF2 __berylium fluoride___ ________ p3 shape: _pyramidal___ BH3 ___Boron hydride_____ ________ p2 shape: _bent line____ PH3 __Phosphorus tri hydride__ ________ p shape: __Line______ Cl2 __Chlorine__________ ________ 1. 2. 3. 4. ___hydrogen_____ ________ A covalent bond is created when _______(#) electrons are shared. A triple covalent bonds has _______(#) electrons shared. Ammonia (NH3) makes a_____________shape and is ___________(polar or non-polar). Oxygen has ________(#) pairs of unbonding electrons in a water molecule, these electrons are called ________ pairs. Vocabulary – know definitions and be able to use in the context of a question: Covalent Bond – Ionic Bond – Electron Dot Structures – Octet Rule – Electronegativity – VSPER Selected Answers: 1. Two 2. Six 3. Pyramidal, polar 4. Two, lone pairs