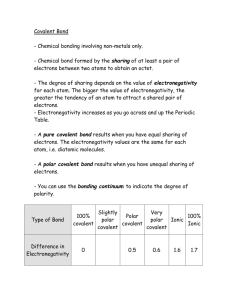
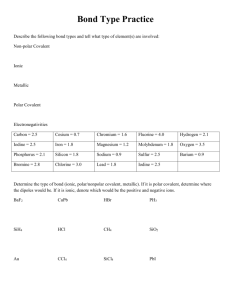
p.11 - Ms Beaucage
advertisement
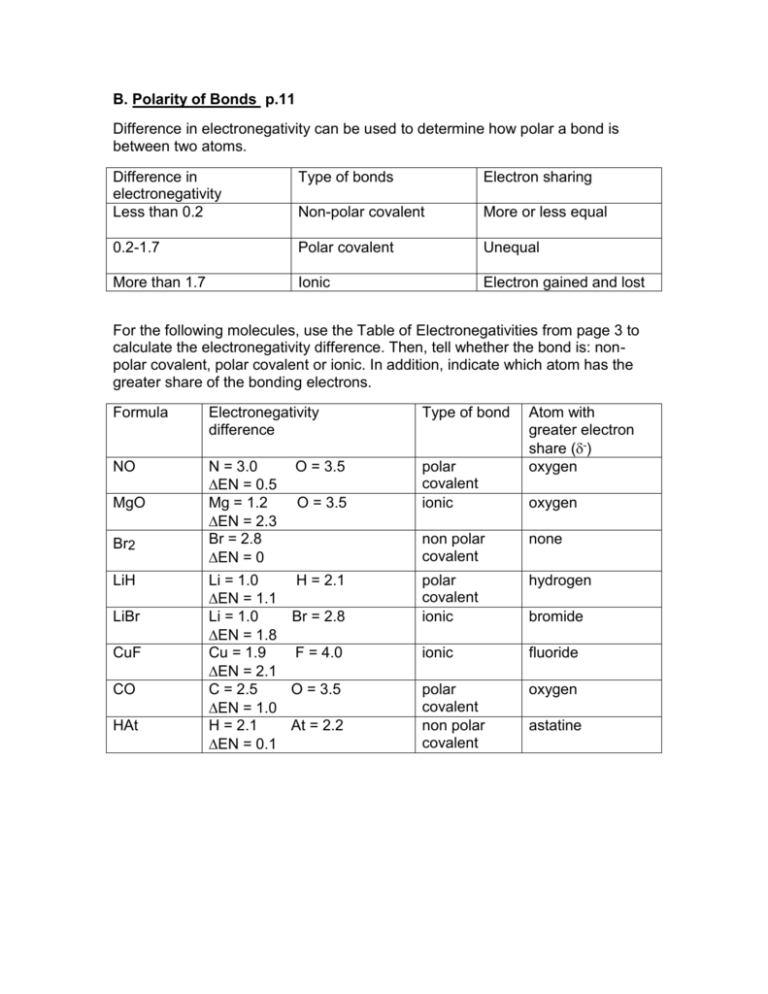
B. Polarity of Bonds p.11 Difference in electronegativity can be used to determine how polar a bond is between two atoms. Difference in electronegativity Less than 0.2 Type of bonds Electron sharing Non-polar covalent More or less equal 0.2-1.7 Polar covalent Unequal More than 1.7 Ionic Electron gained and lost For the following molecules, use the Table of Electronegativities from page 3 to calculate the electronegativity difference. Then, tell whether the bond is: nonpolar covalent, polar covalent or ionic. In addition, indicate which atom has the greater share of the bonding electrons. Formula Electronegativity difference Type of bond NO N = 3.0 O = 3.5 EN = 0.5 Mg = 1.2 O = 3.5 EN = 2.3 Br = 2.8 EN = 0 Li = 1.0 H = 2.1 EN = 1.1 Li = 1.0 Br = 2.8 EN = 1.8 Cu = 1.9 F = 4.0 EN = 2.1 C = 2.5 O = 3.5 EN = 1.0 H = 2.1 At = 2.2 EN = 0.1 polar covalent ionic MgO Br2 LiH LiBr CuF CO HAt Atom with greater electron share (-) oxygen oxygen non polar covalent none polar covalent ionic hydrogen ionic fluoride polar covalent non polar covalent oxygen bromide astatine