Ch 8 Biochemistry / Organic Chemistry Vocabulary
advertisement

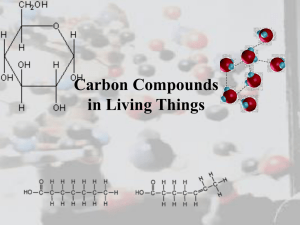
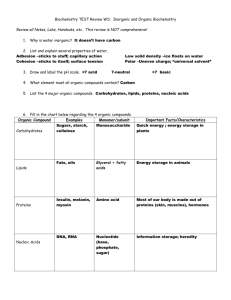
Student Name: _______________________________ Mrs. Lee – 8th Grade Physical Science Period: ______ Date: __________ Page 1 Organic Chemistry and Biochemistry The bonding characteristics of carbon allow the formation of many different organic molecules of varied sizes, shapes, and chemical properties and provide the biochemical basis of life. a) Students know large molecules (polymers), such as proteins, nucleic acids, and starch, are formed by repetitive combinations of simple subunits. b) Students know the bonding characteristics of carbon that result in the formation of a large variety of structures ranging from simple hydrocarbons to complex polymers and biological molecules. c) Students know amino acids are the building blocks of proteins. Vocabulary Words Organic compounds: They are complex molecules made by organisms containing carbon as their “backbone” & usually hydrogen (carbohydrates, proteins, lipids, nucleic acids). The ability of carbon to bond to other elements, and to allow different arrangements of atoms contributes to the diversity of carbon compounds. Carbon, hydrogen, nitrogen, oxygen, phosphorus and sulfur (CHNOPS) makes up about 99% of the mass of all living organisms. Hydrocarbons: They are molecules composed of hydrogen and carbon. They are important because they are the backbones of other organic compounds. Amino acids: They are the building blocks of proteins. There are 20 different amino acids but they all have the same basic “backbone” structure. Proteins: They are long chains of amino acid units that are the main molecules from which living things are constructed. They have many functions in the body: structural components, transport aids, enzymes, cell signals, etc. Lipids: They are organic molecules used to form cellular and organelle membranes, the sheaths surrounding nerve fibers, and certain hormones. They include fats, a long-term energy source. They are insoluble in water (repel water). Examples are oils, butter, lard, etc. They are not as easily metabolized as carbohydrates, yet they are a more effective means of storage. Example: one gram of fat provides two times the energy of one gram of carbohydrate. Carbohydrates: The primary energy source for living things. They are composed of carbon, hydrogen, and oxygen. Serve as energy sources and provide structural support, as in the cell wall of plants. Member of a large class of chemical compounds that includes sugars, starches, cellulose, and related compounds. They are produced naturally by green plants from carbon dioxide and water. As essential nutrients, they are the human body’s main source of both quick and sustained energy. Starch: It plays a vital role in the biochemistry of both plants and animals. It is made in green plants by photosynthesis and is one of the main forms in which plants store food. Animals obtain starch from plants and store it as glycogen. Both plants and animals convert starch to glucose when energy is needed. Nucleic acids: They are large molecules comprised of nucleotides that carry the genetic code. They are found in the nucleus of the cell. Specifically, they are DNA (deoxyribonucleic acid) and RNA (ribonucleic acid). Nucleotide: This is the unit that makes up nucleic acid. It contains a nitrogen base, a phosphate group, and a carbohydrate (sugar) molecule. The 4 possible nucleotide bases for DNA are 1) Adenine, 2) Guanine, 3) Cytosine, & 4) Thymine. The 4 possible nucleotide bases for RNA are 1) Adenine, 2) Guanine, 3) Cytosine, & 4) Uracil. Chemical formula: A combination of chemical symbols and numbers to represent a substance. Structural formula: A formula that indicates the location of the atoms, groups, or ions relative to one another in a molecule and that indicates the number and location of chemical bonds. Check out http://www.borg.com/~lubehawk/biochem.htm 3/5/07 Student Name: _______________________________ Mrs. Lee – 8th Grade Physical Science Period: ______ Date: __________ Page 2 Sample Structure of an Amino Acid: Sample Structure of a Protein: Sample Structure of a Carbohydrate: Glucose Sample Structure of a Lipid: The monomer for a lipid is a hydrocarbon (carbon atom with 2 hydrogen atoms bonded to it). Hydrocarbons can link together to form long chains. Fat: glycerol + 3 fatty acids Check out http://www.borg.com/~lubehawk/biochem.htm 3/5/07