Exam 1 in class
advertisement

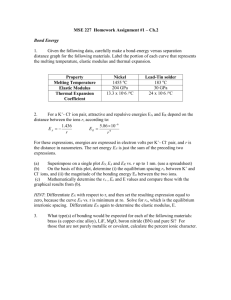
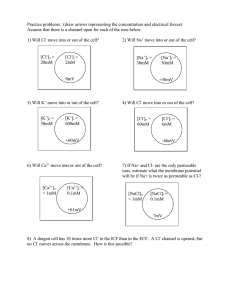
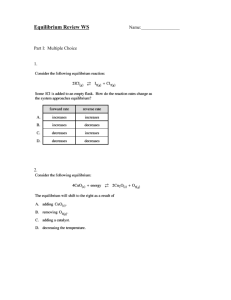
Dr. Casey Exam 1 Spring 2005 ENVS 602 Name: ______________________ Signature: ______________________ Honor Statement: By signing this exam, you indicate that you have neither given nor received aid from any unauthorized source. For this exam you may use a general chemistry book for reference. No other resources are allowed. Answer each question in the space provided. If you need more space, use the back of the sheet. All work must be shown to receive partial credit. Useful Information [G] = KH * PG 1 atm = 101,325 Pa 1 atm = 760 mm Hg = 760 torr 1. a. Given the following reactions, which complex will form to a greater extent? Explain why. 1) Cu2+ + Cl- CuCl+ log Kf = 0.20 2) Cu2+ + Br- CuBr+ log Kf = -0.03 b. Given this additional reaction (3), determine the equilibrium constant for reaction 4. 3) Cu2+ + 2Cl- CuCl2 4) CuCl+ + Cl- CuCl2 log Kf = -0.26 2. Consider all of the species related to Cu and Cl from the question above. In solution, a dynamic equilibrium would be established between all of these species: Cu2+ CuCl+ CuCl2 a. What does dynamic equilibrium mean in this context? b. Suppose that after this equilibrium is established, more Cl is added to the system. What will happen to the concentration of each of the Cu species? c. Suppose that the extra Cl- added in part b was isotopically labeled (most Cl- ions have a mass of 35, these labeled ions have a mass of 37). This means that we can follow these extra Cl- ions through the system. Given that this system will establish a dynamic equilibrium, comment on the distribution of 37Cl- between the Cu species and the uncomplexed Cl-. 3. Runoff water from a mine tailings pile is channeled through a shallow ditch toward a constructed wetland. The ditch contains a gravel bottom and the water is well aerated. The constructed wetland is vegetated with cattails (Typha latifola) and the sediment contains a thick layer of decaying vegetation from the prior year’s growth. Water flows mainly through the wetland subsurface and is then released as surface water into an adjacent stream. The inlet and outlet of the wetland were monitored for total Cd and SO42-. Cd (g/L) Inlet Outlet SO42- (mg/L) 473 34 136 83 a. Explain the factors affecting redox status in the channel and the wetland sediments. b. Explain why passage through the wetland caused the observed changes in these two constituents. 4. a. Dissolved organic matter isolated from Site A is assessed for the number of Cu binding sites per mg C under different conditions. Why do these values differ? pH 5.5 pH 8.3 [L] = 1.98 x 10-6 mol/mg C [L] = 8.32 x 10-6 mol/mg C b. Dissolved organic matter was also isolated from Site B and was found to have the following concentration of Cu binding sites per mg C. Why are there differences between sites? pH 5.5 [L] = 0.63 x 10-6 mol/mg C 5. Hydrated metal ions, M(H2O)6n+, can act as acids. Explain how the acidity occurs and which of the following would be more acidic. Fe(H2O)62+ Fe(H2O)63+