UV-Vis Molecular Absorption Spectrometry
advertisement

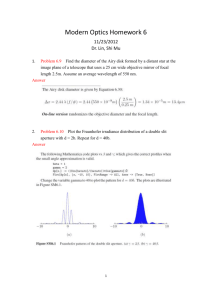
UV-Visible Molecular Absorption Spectrometry Mainly Chapters 7 and 13, a little of Chapter 6 and 14 also. Based on an increase in electronic energy (Eelectronic) from the absorption of a photon in the UV or visible region, as previously discussed. Absorption spectrophotometry is the single most common method for quantitative analysis of molecules, but… Beer’s Law has its limitations (Section 13B). 1. Already know about linear range, for any instrumental method. In the derivation of Beer’s Law, it is assumed that solutes act independently. If the solute conc. is too high, then that is not a good assumption and the absorptivity changes. 2. In the derivation of Beer’s Law monochromatic incident radiation is assumed to irradiate the sample. If a source is used that puts out light of all wavelengths (called a continuous source, used in this type of spectrometry), then Beer’s Law does not apply. To illustrate this 2nd point, consider a beam of incident radiation on an absorbing sample consisting of 2 wavelengths 1 3. Stray Light: Minimize by closing the cover! Cannot eliminate. 2 UV-Vis Molecular Absorption Instrumentation (Section 13D + parts of Chapters 6 & 7) First – sources. A continuous source that emits all wavelengths of radiation in the region is used. Mostly – Blackbody radiators. When a conducting solid is heated, it will emit electromagnetic radiation (incandescence). 1. The total amount of light energy increases with increasing temperature. 2. The spectral intensity shifts to higher energies with increasing temperature. The W filament lamp is the most common source for visible (and near-infrared) spectrometers. (~2900K) The W/halogen lamp can operate at a higher temperature, and can be used as a UV source (if housed in quartz which does not absorb UV light, rather than glass which does). ~3500K. They are also superior visible sources. 3 The continuous spectral output of a blackbody radiator can be understood qualitatively from the MO diagram of a solid conductor. Compare this to the Atomic orbital diagram of Na discussed earlier. A more common UV source is the D2 lamp which provides a continual spectral output from 190-400 nm. QM is necessary to understand how these work. LED for smaller instruments like the OOI spectrometer we have often been using. LED does not produce a continuous output, so how does it put out a continuous spectrum of white light? If the spectrometer’s source emits a continuous range of wavelengths of light, and the goal is to obtain a spectrum of absorbance as a function of wavelength, there must be a way to select a wavelength, or range of wavelengths, for sample irradiation. Second – Wavelength selectors. Section 7C Ideally – output from a wavelength selector is a single wavelength. Reality – output from a wavelength selector is a band of wavelengths. The narrower the band, the better the wavelength selector, the greater the spectral resolution attainable. 4 Wavelength selectors can be either filters or monochromators. We will discuss monochromators exclusively. In any case the effective bandwidth is one important defining factor of a wavelength selector’s performance, defining its resolution. Monochromators can be based on the use of prisms, which work on the principal of refraction, or gratings, which work on the principal of diffraction. You can see that a traditional monochromator consists of more than a grating or a prism, but these are the parts of a wavelength selector most important to selecting a band of wavelengths from a continuous source. The next figure shows why diffraction gratings are superior to prisms for most applications. 5 Now a few specifics about grating monochromators. Function to disperse different wavelengths of light at different angles. Angular dispersion: dr/dλ Remember from earlier: This would be a transmission grating, where it was shown that the following conditions result in constructive interference: nλ = d sinθ where d is the distance between the holes in the grating Monochromators invariably use a reflection grating, where closely spaced graves are cut out of a mirrored face. In this case constructive interference occurs when nλ = d(sin i + sin r) i is the angle of incidence, r is the angle of reflection. d is defined at left, and n is an integer value (diffraction order) 6 Differentiation of the above equation at a constant angle of incidence: While the angular dispersion is important, the linear dispersion D is more relevant, since it refers to the variation in wavelength along the focal plane (AB in figure of grating monochromator) Most important is the inverse of the linear dispersion, the reciprocal linear dispersion D-1 This to a large extent determines the spectrometer’s resolution. The ability to distinguish absorbances at different wavelengths close to one another. A grating monochromator’s resolving power depends largely on d – the space between grating blazes f – the monochromator’s focal length n – the diffraction order Say a conventional grating has 1200 blazes/mm and a focal length of 0.5 m. (Both of these numbers are pretty standard). Find D-1 for n = 1 Find D-1 for n = 2 Find D-1 for a grating with 600 blazes/mm Will a large D-1 or a small D-1 provide better resolution? 7 Why is spectral resolution important? For one: the S/N lab was meant to illustrate this with the vibrational spectrum of CO2. Here is a second illustration for a vapor phase UV spectrum of benzene Resolution will be revisited soon. First, a discussion of the last major instrument component of UV-Visible molecular absorbance spectrometers. Portions of Section 7E, detectors. In the UV-Vis region we discuss 3 types, the first 2 are very similar in principle but have very different performance characteristics. 1. Vacuum Phototube 2. Photomultiplier tube Both of these are “photoemissive” devices. Shine light on them, and they emit electrons. 3. Multichannel photon transducers. Shine light on these, and they conduct electricity. Advantage of smaller size and can construct instruments with a different design as a result. 8 The photoemissive detectors work on the principle of Einstein’s photoelectric effect (Section 6C). Here is a vacuum phototube. The cathode is coated with a low ionization energy material. 9 117 = K-Cs-Sb, S11 = Cs3Sb Vacuum phototube: 1 photon > ionization energy of photocathode => 1 photoelectron emitted. Photomultiplier tube: 1 photon > ionization energy of photocathode => ~106 photoelectrons emitted. PMT – much greater sensitivity. Much lower light levels give measurable signals. In a PMT there is still a photocathode, but between that and the anode are a series of dynodes. 10 If at each dynode 5 e- are emitted for each electron that strikes it, for the 9 diode arrangement above the PMT current gain = 59 Dynodes are irreversibly damaged by high intensity light, which is why we are paranoid when using the fluorescence spectrometer. These 2 photoemissive detectors are fine, but they are large. The usefulness of a very small detector will be shown after a brief discussion of the basic principles of how one works. Small photoconductive detectors are based on semiconductors. 11 Since each detector is about 25μm, a small instrument can fit many detectors. This allows for a different type of instrument. Types of Instruments – Section 13D-2 Single Channel instruments use a single large detector like a vacuum phototube or a photomultiplier. These single channel instruments can be single beam like the Spectronic 20 used in the instrument components lab. These single channel instruments can also be double beam like the Shimadzu UV160 12 The small semiconductor detectors afford the capability of building a multichannel instrument. like the Ocean Optics single beam Array Spectrometer. Now as promised, spectral resolution revisited. First – single channel instruments (Section 7-C3), then multichannel instruments. Spectral resolution is determined by the monochromator. Thus far only the grating has been discussed, and its D-1. To understand resolution we must also think about entrance and exit slits. 13 For a single channel instrument almost invariably: The size of the entrance slit width = size of exit slit width Different wavelengths are brought to the exit slit by rotating the grating Consider the following scenario if we have monochromatic source input into the monochromator, say from a Na vapor lamp. 1. Monochromator illuminated with line source λo = 589 nm 2. Entrance slit width = exit slit width = 1 mm 3. Monochromator D-1 = 20 nm/mm D-1 = dλ/dy = range of wavelengths spread over the distance dy along the exit slit focal plane. 1. When monochromator set to 589 nm, entrance slit image fills exit slit – maximum signal intensity. 2. When monochromator set to 579 or 599 nm, entrance slit image half fills exit slit – half of maximum signal intensity. 3. When monochromator set to 569 or 609 nm, entrance slit image misses exit slit – no signal intensity. This is the origin of the triangular slit function shown above. Little sense in illuminating a monochromator with monochromatic light… Consider the more realistic scenario 14 1. Monochromator illuminated with polychromatic light 2. Entrance slit width = Exit slit width = 1 mm 3. Monochromator D-1 = 20 nm/mm Now – For every λ present there is a triangular distribution of energies exiting the monochromator. When the monochromator is set to 589 nm – what range of wavelengths are passed through the exit slit? Setting the monochromator to 589 nm: 100% of source power at 589 nm passes through 50% of source power at 579, 599 nm passes through etc. The sample is illuminated with a polychromatic band of light of various wavelengths each with varying intensity! To define the width of the wavelength band, we go back to the effective bandwidth, FWHH, now also called the spectral slit width (S) S = W x D-1 where W = physical slit width 15 Spectral slit width or effective bandwidth defines spectrometer resolution affects the applicability of Beer’s Law affects S/N affects the ability to acquire detailed spectral information Earlier the deviations from Beer’s law due to polychromatic radiation were addressed. If the monochromator’s effective bandwidth < 10% of the width of the FWHH of the absorption band, then Beer’s law is obeyed. Since absorption bands in UV-Vis absorption in liquids are so broad, this is usually not a problem. Spectral resolution with a polychromator (i.e. a multichannel instrument) must be looked at somewhat differently. Here the entrance slit width is fixed such that the image from the continuous source illuminates the entire detector array. Commonly, 1024 detectors are lined up in a 1-D array. [If the detectors are 0.025 mm apart, this many detectors fits along a line of 25.6 mm (about 1 inch)!] Since there is no exit slit there is no slit function as with a single channel instrument. If there are 1024 detectors over a given spectral range Δλ, say 800 nm (200 nm 1000 nm) then the spectral resolution is given by the range of wavelengths that a single detector is sensing. 16 Res. = 800 nm/1024 detectors = 0.78 nm/detector The range of wavelengths distributed in a given distance along the focal plane is still dependent on D-1. Advantages of multichannel instruments: No moving parts, rapid spectral acquisition (signal averaging), enhanced source throughput. Disadvantage of multichannel instruments: spectral resolution is not variable. Finally a little on applications of UV-visible absorption spectrometry (Ch. 14). The absorption of UV/visible light generally results from excitation of bonding electrons. In organic compounds, useful transitions are n π* and π π*. Compounds with useful transitions are said to contain chromophores. Transitions below 200 nm are not useful (vacuum UV). You would think that the wavelengths of absorption bands could be correlated with the types of bonds and functional groups in a compound. In theory that is correct. In practical terms, UV-Vis molecular absorption spectrometry is almost totally useless for qualitative analyses. By far the most important application for this type of spectroscopy is for quantitative analysis. 17 Chapter 6 – Wave & particle properties of EM radiation. Diffraction. Interaction of radiation with matter, absorption, emission. Line spectra, continuous spectra, atomic/molecular absorption and emission. Quantitative aspects of absorption and emission measurements. Problems/Questions 1e,f,g,h,l, 2, 3, 7, 8, 9a, 13, 14, 15 Chapter 7 – General designs of optical instruments, UV-Vis continuous sources, grating monochromators and performance characteristics and resolution, UV-Vis detectors (radiation transducers), optical Fourier transform spectroscopy. Problems/Questions 1, 3, 4, 5, 8, 19a, 20, Chapter 13 – Transmittance, absorbance, Beer’s Law and limitations/deviations, slit widths and effects on spectra, instrumentation (sources, types of instruments). Problems/Questions 1, 2, 5, 7, 8, 9, 13b,g,h, 17, 18, 22, 23 18