Analysis Questions
advertisement

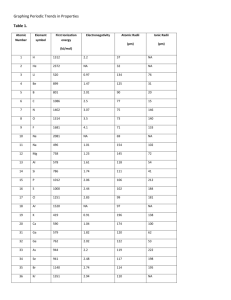
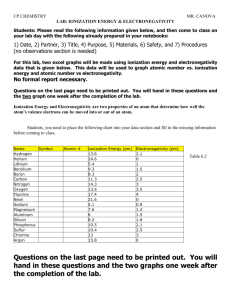
Name_____________________________ Class_____________________________ Date______________________________ Discovering Periodic Trends Graphing Trends 1. Define ionization energy, electronegativity, atomic radius, & ionic radius. 2. Using the data in Table 1: Graph ionization energy (y-axis) vs. atomic number (x-axis). Identify each data point with the element’s symbol. Connect your data points in a “dot-to-dot” manner. Graph electronegativity (y-axis) vs. atomic number (x-axis). Identify each data point with the element’s symbol. Connect your data points in a “dot-to-dot” manner. Graph atomic radius (y-axis) vs. atomic number (x-axis). Identify each data point with the element’s symbol. Connect your data points in a “dot-to-dot” manner. Analysis Questions 1. a. Make a list of the elements that have the greatest ionization energies (those that appear at the peaks of your graph) and a list of the elements that have the smallest ionization energies (those at the “valleys” of your graph). b. Is there a relationship among the elements in each list and their positions on the periodic table? c. Describe the general trend in ionization energy down a group and across a period. 2. a. Make a list of the elements that have the greatest electronegativities (those that appear at the peaks of your graph) and a list of the elements that have the smallest electronegativities (those at the “valleys” of your graph). b. Is there a relationship among the elements in each list and their positions on the periodic table? c. Describe the general trend in electronegativity down a group and across a period. 3. Describe the general trend in atomic radius down a group and across a period. 4. Is a positive ion larger or smaller than its neutral atom? Give an explanation for this observation considering the number of protons and electrons that remain after an ion is formed and that protons and electrons attract since they are oppositely charged. (Hint: use a particular atom and its ion as an example in your explanation). 5. Is a negative ion larger or smaller than its neutral atom? Give an explanation for this observation considering the number of protons and electrons that remain after an ion is formed and that protons and electrons attract since they are oppositely charged. (Hint: use a particular atom and its ion as an example in your explanation).