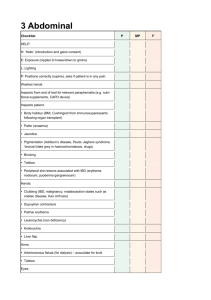
Enzymatic activity measurements of tissues
advertisement

ELECTRONIC SUPPLEMANTARY MATERIAL Enzymatic activity measurements of tissues Preliminary enzymatic assay were performed to measure PO activity in haemolymph, abdomen, abdomen 5 devoid of the digestive tract and thorax. PO activity was higher in haemolymph and abdomen devoid of the digestive tract compared to abdomen and thorax. The difference in PO activity between abdomen and abdomen devoid of the digestive tract may be due to the presence of inhibitors or inhibitory enzymes in the digestive tract. For our experiment, PO activity was measured on abdomen devoid of its digestive tract instead of haemolymph because the variability in the activity was lower in the abdomen (unpublished 10 data), probably due to a high variance in the volume of haemolymph between individuals. The age dependency of PO activity in haemolymph (Schmid et al. 2007; Wilson-Rich et al. 2008) was also observed in our measurements on abdomen devoid of the digestive tract (unpublished data). Abdomens and heads were dissected and immediately placed in ice cooled tubes and frozen at -20°C until homogenization. The different biological compartments were homogenized at 4°C, using a TissueLyser 15 (Qiagen, Courtaboeuf, France), in 10% (w/v) phosphate buffer (80 mM NaH2PO4/Na2HPO4, 20 mM NaCl, 1% (w/v) Triton X-100, pH 7.4) containing a mixture of 2 mg/ml of antipain, leupeptin and pepstatin A, 25 units/ml of aprotinin, 0.1 mg/ml of trypsin inhibitor as antiproteolytic agents. The buffer enabled to control the ph and protein solubility and digest cellular membranes. The homogenates were then centrifuged at 15,000 g for 20 min at 4°C. The supernatant was used for analysis of enzymatic 20 activities and protein contents. PO activity PO activity assays are based on the conversion of L-Dopa (3,4-Dihydroxy-L-phenylalanine) to melanin. Samples were combined with extraction buffer (80 mM NaH2PO4/Na2HPO4, 20 mM NaCl, 1% (w/v) 25 Triton X-100) prior to enzymatic measurement. Tissue homogenates were diluted using a 2:3 extract ratio. The reaction mixture consisted of 90 μL of distilled water and 20 μL of sodium phosphate buffer 1 (100 mM NaH2PO4, 200 mM NaCl, pH 7.2), to which 50 μL of freshly extracted sample was added. The microplate was incubated 5 min at 37°C before adding 40 μL of the enzymatic substrate L-Dopa (2 mg/mL) to each well. PO activity was quantified by recording the change in sample absorbance at 490 nm for 10 min. 5 GOX activity GOX catalyses the conversion of β-D-glucose and O2 into gluconic acid and H2O2. H2O2, in the presence of o-dianisidine, can in turn be converted by peroxidase into oxidised o-dianisidine, a spectrophotometrically active compound. The reaction mixture consisted of 100 μL of distilled water and 10 50 μL of potassium phosphate buffer (500 mM KH2PO4/K2HPO4, pH 7.0), containing 20 μL of glucose (100 mM) and 10 μL of horseradish peroxidase (2.5 U). Glucose solution was prepared 1 h in advance to allow β-mutarotation of the glucose and the reaction mixture was incubated for 10 min at 37°C prior to absorbance measurement. 10 μL of freshly homogenized sample was incubated for 10 min at 37°C in the microplate. Then, 20 μL of o-dianisidine (3 mM) was extemporaneously added to the solution. GOX 15 activity was quantified by recording the change in sample absorbance at 430 nm for 1.5 h. Analyses were performed in triplicates for each sample and enzyme activity. Absorbance data were obtained using a BioTek Synergy HT100 plate reader (BioTek Instruments, Colmar, France) and enzyme activities were measured as the slope of the linear phase of reaction. 20 References Schmid, M.R., Brockmann, A., Perk, C.W., Stanley, D.W. & Tautz, J. 2007 Adult honeybees (Apis mellifera L.) abandon hemocytic, but not phenoloxidase-based immunity. J. Insect. Physiol. 54 Wilson-Rich, N., Dres, S.T. & Starks, P.T. 2008 The ontogeny of immunity: development of innate 25 immune strength in the honey bee (Apis mellifera). J. Insect Physiol. 54 1392-9. 2