Prevention and
management of preeclampsia and
eclampsia
A Reference Manual for
Health Care Providers
Copyright © 2011, Jhpiego. All rights reserved. The material in this document may be freely
used for educational or noncommercial purposes, provided that the material is accompanied
by an acknowledgement line.
Suggested citation: MCHIP. Prevention of eclampsia: A Reference Manual for Health Care
Providers. Baltimore: Jhpiego; 2011.
Prevention and management of pre-eclampsia
and eclampsia
A Reference Manual for Health Care Providers
2011
Maternal and Child Health Integrated Project
(MCHIP)
This project is made possible through support provided to MCHIP by the Office of Health, Infectious Diseases and Nutrition, Bureau
for Global Health, US Agency for International Development, under the Cooperative Agreement No. GHS-A-00-08-00002-00.
MCHIP is implemented by a collaborative effort between Jhpiego, Save the Children, John Snow, Inc (JSI), MACRO, Johns Hopkins
University Institute for International Programs (IIP), Program for Appropriate Technology for Health (PATH), Broad Branch
Associates (BBA), Population Services International (PSI), Collaborating Organizations: Communication Initiative (CI), CORE, and
others.
iv
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
Table of contents
Introduction .............................................................................................................. 1
Understanding pre-eclampsia and eclampsia.................................................................. 5
Pathophysiology ................................................................................................... 5
Epidemiology of pre-eclampsia and eclampsia .......................................................... 6
Factors influencing maternal and perinatal outcomes ................................................ 7
Morbidity and mortality associated with pre-eclampsia and eclampsia ......................... 8
Identifying pre-eclampsia .......................................................................................... 11
Introduction ...................................................................................................... 11
Definition .......................................................................................................... 11
Screening .......................................................................................................... 12
Detecting hypertensive disorders in pregnancy ...................................................... 16
Prevention of pre-eclampsia and/or eclampsia ............................................................. 21
Primary prevention of pre-eclampsia..................................................................... 21
Secondary prevention (screening and detection) .................................................... 22
Tertiary prevention (management of severe pre-eclampsia and eclampsia) ............... 23
Overview of interventions to prevent pre-eclampsia and eclampsia........................... 27
Management of pre-eclampsia and eclampsia .............................................................. 29
Introduction ...................................................................................................... 29
Gestational hypertension ..................................................................................... 29
Mild pre-eclampsia - Gestation less than 37 weeks ................................................. 30
Mild pre-eclampsia - Gestation of 37 complete weeks or more ................................. 31
Severe pre-eclampsia and eclampsia .................................................................... 31
Postpartum care................................................................................................. 38
Referral for tertiary level care .............................................................................. 38
Management during a convulsion / fit ......................................................................... 39
Stages of an eclamptic fit .................................................................................... 39
Management during a convulsion ......................................................................... 40
Differential diagnosis of convulsions ..................................................................... 41
Birth preparedness and complication readiness ............................................................ 45
Birth-preparedness plan ...................................................................................... 45
Complication-readiness plan ................................................................................ 46
References .............................................................................................................. 49
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
v
Acknowledgements
Susheela Engelbrecht led development of the learning materials, with technical assistance
and feedback from members of the MCHIP Training and Quality Assurance Task Force, one
of the five Task Forces formed under the Pre-Eclampsia/Eclampsia Technical Working Group.
Members of the task force include Patricia Gomez, Diane Sawchuck, Peter von Dadelszen,
Abdelhadi Eltahir, Frances Ganges, Ann Davenport, Deborah Armbruster, Nahed Matta,
Jeffrey Smith, Annette Briley, and Bridget Lynch. The writing team is grateful to Ahmet
Metin Gulmezoglu for review of this draft.
About MCHIP
vi
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
Acronyms
BP
blood pressure
BPP
birth preparedness plan
CRP
complication readiness plan
dBP
diastolic blood pressure
DIC
disseminated intravascular coagulation
HELLP
Hemolysis, ELevated Liver enzymes, and low Platelet count
syndrome
HIP
hypertension in pregnancy
IUGR
intrauterine growth restriction
Magpie Trial
magnesium sulfate for prevention of eclampsia trial
MAP
mean arterial pressure
MCHIP
maternal and child health integrated project
MDG
Millennium Development Goals
RCT
randomized controlled trial
sBP
systolic blood pressure
STI
sexually transmitted infections
UTI
urinary tract infection
USAID
United States Agency for International Development
WHO
World Health Organization
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
vii
viii
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
Reference manual
Introduction
Efforts such as the Safe Motherhood Initiative and the World Health Organization (WHO)
Making Pregnancy Safer Division and strategies to meet the United Nations Millennium
Development Goals (MDG) are supporting worldwide activities to reduce maternal and
newborn mortality. Despite these efforts, hundreds of thousands of women and babies die
or become disabled due to complications of pregnancy and childbirth every year (WHO,
1994).
Women die from a wide range of complications in pregnancy, childbirth or the postpartum
period. Most of these complications develop because of their pregnant status and some
because pregnancy aggravated an existing disease. The four major causes are severe
bleeding (mostly postpartum hemorrhage), infections (also mostly in the immediate
postpartum), hypertensive disorders in pregnancy (eclampsia), and obstructed labor. New
estimates show that the leading causes of maternal deaths are hemorrhage and
hypertension, which together account for more than half of maternal deaths (figure xx)
(WHO and UNICEF, 2010). Indirect causes, which include deaths due to conditions such as
malaria, HIV/AIDS and cardiac diseases, account for about one fifth of maternal deaths.
Regional estimates show that hemorrhage and hypertension are among the top three
causes of deaths in both South Asia and Sub-Saharan Africa, where the majority of
maternal deaths occur (WHO and UNICEF, 2010). Pre-eclampsia and eclampsia may also
occur in the immediate post-partum period and is referred to as "postpartum preeclampsia." The most dangerous time for the woman is the 24–48 hours postpartum and
careful attention should be paid to pre-eclampsia signs and symptoms (Manjuluri et al,
2005).
Figure xx. Global estimates of the causes of maternal deaths, 1997-2007
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
1
Ten percent of all pregnancies are complicated by hypertension (HTN) (Craici et al, 2008).
Pre-eclampsia and eclampsia account for about half of these cases worldwide and have been
recognized and described for years despite the general lack of understanding of the disease
(Roberts et al, 2001). The fetal mortality rate associated with pre-eclampsia and eclampsia
varies from 13-30%, primarily due to premature delivery and its complications (Gabbe,
2007). Placental infarcts, abruptio placentae, and intrauterine growth restriction also
contribute to fetal demise (Gabbe, 2007). Maternal death risk associated with preeclampsia and eclampsia is approximately 1.8%, in high resource settings, and up to 14%
in settings with low resources and lack of facilities required for supportive
management. Higher mortality rates are associated with women who have multiple
convulsions/fits outside the hospital and those without antenatal care (Rivers, 1996).
Fortunately, simple, low-cost interventions are available to prevent most cases of eclampsia
and to manage them when they occur. Providers at all levels must be able to identify preeclampsia and eclampsia and know how to respond. Timely diagnosis and effective initial
management can reduce morbidity and the risk of maternal, fetal, and newborns deaths
associated with severe pre-eclampsia and eclampsia. Once providers identify pre-eclampsia
and eclampsia, they must be competent to provide effective initial management and ensure
timely referral for cases that cannot be managed at their facility level.
To facilitate access to important maternal technologies, countries must have the political will
and ensure that the following are in place:
National guidelines that reflect state-of-the-art and evidence-based interventions for
prevention, identification and management of pre-eclampsia and eclampsia
Policies that promote access to important technologies for prevention, identification
and management of pre-eclampsia and eclampsia at all levels along the continuum of
care
Training infrastructure that promotes pre-service education and periodic updates and
refresher training for all health workers in prevention, identification and management
of pre-eclampsia and eclampsia at all levels along the continuum of care
Service delivery systems that ensure quality and promptness of response for women
with severe pre-eclampsia/ eclampsia
Logistics systems that ensure availability of necessary resources (equipment,
supplies, medications - magnesium sulphate and calcium gluconate) and
consumables for infection prevention and injection safety
Supervision and monitoring systems that assure quality and ensure transfer of
learning to the work site
A service delivery model that promotes education and knowledge sharing with
women, families and communities about the risks, signs and symptoms, and how to
respond when the woman presents with signs or symptoms of pre-eclampsia and
eclampsia
Links between communities and health care facilities that ensure that the woman
receives timely and appropriate care
Ongoing research in various settings continues to identify the best approaches for
preventing and managing eclampsia and its complications. By developing national
guidelines, training health care providers, improving work environments, and supporting the
development of improved access to care, more women will have access to life-saving
interventions that reduce morbidity and mortality associated with pre-eclampsia and
eclampsia.
2
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
Reference manual
About the learning materials
MCHIP developed a learning package on the prevention and management of pre-eclampsia
and eclampsia consisting of a reference manual, participant’s notebook, and facilitator’s
guide. This learning package was developed for use by clinical health workers, nurses,
midwives, and physicians providing care during pregnancy, childbirth and the postpartum.
These documents comprise a set and should be used together. These resources are
distinguished within the series by a corresponding icon located at the top of the right hand
page:
Reference manual
Facilitator’s guide
Participant’s notebook
This course is designed to be utilized for in-service training, with the overall objective of
providing updates about prevention and management of pre-eclampsia and eclampsia use
to equip nurses, midwives, physicians, trainers and clinical health workers to carry out the
following:
Provide safe, respectful, culturally sensitive, and friendly care to women, newborns, and
their families. Women and families will then be more likely to utilize the health care
system with confidence because they know they will receive competent, compassionate
care.
Follow an evidence-based protocol for prevention, identification, and management of
pre-eclampsia and eclampsia, including clear guidelines on when to refer women with
complications, ensuring timely action is taken.
Provide greater protection from infection for their clients and exercise of universal
precautions for themselves.
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
3
4
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
Reference manual
Understanding pre-eclampsia and eclampsia
Pathophysiology
Cause
Pre-eclampsia is a pregnancy-specific syndrome, recognized, even by Hippocrates, as a
leading cause of maternal and perinatal mortality. The condition’s former name, “toxemia of
pregnancy,” was based on a theory that a toxin produced in a pregnant woman’s body
caused the disease. The cause of pre-eclampsia and eclampsia remains unknown, though
experts have proposed multiple theories to explain their cause, resulting in confusion and
myths surrounding both etiology and management. The main etiologic theories
include abnormal trophoblastic invasion, coagulation abnormalities, vascular endothelial
damage, cardiovascular maladaptation, immunologic phenomena, genetic predisposition,
and dietary deficiencies or excess (Craici et al, 2008).
Pathophysiologic changes
In normal pregnancies, blood volume increases 30 to 50%, peripheral vascular resistance
decreases, progesterone induced arterial dilatation occurs, fibrinogen is increased, and
factor XIII (fibrin stabilizing factor) is decreased. The following pathophysiologic changes
are associated with pre-eclampsia and eclampsia:
Blood pressure begins to rise after 20 weeks of pregnancy
Perfusion is decreased to virtually all organs, which is secondary to intense vasospasm
due to an increased sensitivity of the vasculature to any pressor agent
Perfusion to the kidneys is decreased, resulting in sodium retention that leads to loss of
intravascular plasma volume, increased extracellular volume (edema) and increased
sensitivity to pressor agents
Loss of normal vasodilation of uterine arterioles results in decreased placental perfusion
Decreased intravascular volume results in increased viscosity of the blood and a
corresponding rise in hematocrit, and activation of the coagulation cascade, especially
platelets, with microthrombi formation
HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome is
sometimes associated with severe pre-eclampsia and results from activation of the
coagulation cascade.
Disease progression
Pre-eclampsia and eclampsia are part of the same disorder with eclampsia being the severe
form of the syndrome. Gestational hypertension may progress from a mild hypertensive
disorder to a life-threatening condition, as follows:
hypertension without proteinuria or edema
mild pre-eclampsia
severe pre-eclampsia
eclampsia
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
5
While pre-eclampsia is usually a progressive disease, the rate of progression and the
occurrence of catastrophic complications such as eclampsia, cerebrovascular accident,
severe HELLP syndrome, pulmonary edema or renal failure are difficult to predict. In some
cases, mild pre-eclampsia sometimes progresses to severe pre-eclampsia and eclampsia
very suddenly with little or no warning. In other cases, hypertension or proteinuria are
absent when a woman begins having eclamptic convulsions/fits. Eclampsia can occur during
the antepartum, intrapartum, and postpartum periods; and ninety percent of eclampsia
cases occur after 28 weeks' gestation (Gabbe, 2007).
HELLP syndrome is a group of symptoms that occur in pregnant women who have:
H -- hemolysis
EL -- elevated liver enzymes
LP -- low platelet count
HELLP syndrome occurs in approximately 10% of pregnant women with pre-eclampsia or
eclampsia. Many women have high blood pressure and are diagnosed with pre-eclampsia
before they develop HELLP syndrome. However, in some cases, HELLP symptoms are the
first warning of pre-eclampsia and the condition is misdiagnosed as hepatitis, gallbladder
disease, idiopathic thrombocytopenic purpura, or thrombotic thrombocytopenic purpura.
The exact cause of HELLP is unknown, but general activation of the coagulation cascade is
considered the main underlying problem. Fibrin forms cross-linked networks in the small
blood vessels. This leads to a microangiopathic hemolytic anemia: the mesh causes
destruction of red blood cells as if they were being forced through a strainer. Additionally,
platelets are consumed. As the liver appears to be the main site of this process,
downstream liver cells suffer ischemia, leading to periportal necrosis. Other organs can be
similarly affected. HELLP syndrome leads to a variant form of disseminated intravascular
coagulation (DIC), leading to paradoxical bleeding, which can make emergency surgery a
serious challenge.
Symptoms include:
Headache
Nausea and vomiting that continues to get worse
Upper abdominal pain / upper abdominal tenderness on palpation
Vision problems
The woman's liver may hemorrhage and permanent liver damage may occur if delivery is
delayed. Such damage can lead to death. Up to thirty-five percent of women with HELLP
die. The death rate among babies born to mothers with HELLP syndrome varies and
depends on birth weight and the development of the baby's organs, especially the lungs.
Epidemiology of pre-eclampsia and eclampsia
“Risk factors” should not be used to predict complications. The system of
risk categorization, or the “risk approach”, previously used for selecting
women for specialized management is not useful, because evidence shows
that many women categorized as “high risk” do not actually experience a
complication, while many women categorized as “low risk” do.
6
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
Reference manual
Numerous maternal factors may predispose to the disorder; these may be genetic,
behavioral, or environmental. While there are maternal factors predisposing to preeclampsia, a population based approach should be used where all pregnant women are
considered “at risk” of developing pre-eclampsia should be taken.
Factors that may predispose a woman to pre-eclampsia and therefore the risk of eclampsia
include (Roberts et al, 2001; WHO, 1994; WHO and UNICEF, 2010):
Maternal factors
Women with a family history of pre-eclampsia, prior pre-eclampsia and eclampsia
Women with a history of poor outcome of previous pregnancy, including intrauterine
growth restriction, abruptio placentae, or fetal death
Preexisting medical conditions - renal disease, thrombophilias-antiphospholipid
antibody syndrome, protein C deficiency and protein S deficiency, antithrombin deficiency,
vascular and connective tissue disorders, diabetes, gestational diabetes, systemic lupus
erythematosus, increased testosterone, increased insulin resistance, increased blood
homocysteine concentration
Age: Teen pregnancy / Women who are older than 35 years
Lower socioeconomic status
Primigravida (especially young teenagers and women over 35 years)
Primipaternity (first pregnancy with the male partner)
Obese women (BMI > 30)
Women with essential or renal hypertension
Black women or women of African descent
Hydatidiform mole
Fetal factors
Hydrops fetalis
Multifetal gestations
Most traditional risk factors are associated with attributes of the woman or the
pregnancy/fetus. One hypothesis on the causes of pre-eclampsia is immune maladaptation.
Support for this hypothesis comes from epidemiological studies that show that:
the risk for developing pre-eclampsia decreases with the length of exposure to the sperm
that will ultimately fertilize the woman’s egg (Marti et al, 1977)
although pre-eclampsia is generally thought of as a syndrome of first pregnancies, the
protective effect of multiparity is lost with change of partner (Robillard et al, 1999)
men who fathered a pre-eclamptic pregnancy were nearly twice as likely to father a preeclamptic pregnancy in a different woman, regardless of whether she had already had a
pre-eclamptic pregnancy or not (Roberts et al, 2001).
Factors influencing maternal and perinatal outcomes
Pre-eclampsia is a major obstetric problem leading to substantial maternal and perinatal
morbidity and mortality worldwide, especially in low resource settings. Positive outcomes
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
7
depend on how soon a diagnosis is made and how quickly effective treatment is provided.
Maternal and perinatal outcomes in pre-eclampsia depend on maternal, community, and
health care factors (WHO, 2008).
Maternal factors influencing maternal and perinatal outcomes: These include health issues
the woman may have that make the disease worse.
In general, maternal and perinatal outcomes are usually favorable in women with mild
pre-eclampsia developing beyond 36 weeks’ gestation who have no other pre-existing
medical disorders.
By contrast, maternal and perinatal morbidities and mortalities are increased in women
who develop the disorder before 33 weeks’ gestation, in those with pre-existing medical
disorders, and in those receiving care in low resource settings.
Community factors influencing maternal and perinatal outcomes: These are characteristics
of the community that that affect whether and how the woman seeks care.
Lack of awareness about signs and symptoms of pre-eclampsia, severe pre-eclampsia and
eclampsia and the importance of early and regular antenatal care
Transportation barriers particular for obstetric emergencies.
Low socioeconomic status including lack of access to information and low literacy levels
Financial hardship and inability to pay for transport and medical care
Community distrust of health care personnel
Cultural barriers
Health service factors influencing maternal and perinatal outcomes: These are
characteristics of health services that affect the kind of care that women receive.
Inadequate availability and access to antenatal care
Failure to monitor blood pressure and urine during antenatal care
Failure to counsel women and families about dangerous symptoms of severe preeclampsia and the importance of regular antenatal care
Delay in referral of women with symptoms and signs of severe pre-eclampsia or
eclampsia
Lack of a clear-cut management strategy/clinical protocols for dealing with pre-eclampsia
and eclampsia
Inadequately trained staff to treat women with severe eclampsia or eclampsia
Delay in identification and management of severe pre-eclampsia
Lack of proper equipment and drugs to treat pre-eclampsia and eclampsia
Morbidity and mortality associated with pre-eclampsia and
eclampsia
Effects on the woman
These include:
Respiratory problems (asphyxia, aspiration of vomit, pulmonary edema, bronchopneumonia)
Cardiac problems (heart failure)
8
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
Reference manual
Effects on the brain (hemorrhage, thrombosis, edema)
Renal complications (acute kidney failure)
Hepatic disease (liver failure or hemorrhage)
HELLP syndrome (hemolysis, elevated liver enzymes, low platelet count)
Coagulopathy (clotting/coagulation failure)
Visual disturbances (temporary blindness due to edema of the retina)
Injuries during convulsions/fits (fractures)
Abruptio placentae
Stroke
Death
Risk of pre-eclampsia in subsequent pregnancies
Long-term cardiovascular morbidity
The main causes of maternal death in eclampsia are intracerebral hemorrhage, pulmonary
complications, kidney failure, liver failure and failure of more than one organ (e.g. heart +
liver + kidney).
Effects on the fetus
These include:
Intrauterine growth restriction (IUGR)
Preterm delivery
Hypoxia
Neurologic injury
Perinatal death (1–2%)
Long-term cardiovascular morbidity associated with low birthweight (fetal origin of adult
disease)
Hypoxia may cause brain injury if severe or prolonged, and can result in physical and/or
mental disability.
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
9
10
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
Reference manual
Identifying pre-eclampsia
Introduction
Pre-eclampsia is a progressive condition that can lead to stroke, kidney or liver damage,
blood-clotting problems, and pulmonary edema. Eclampsia is commonly defined as the new
onset of convulsions and/or unexplained coma during pregnancy or postpartum in a woman
with signs or symptoms of preeclampsia. However, eclampsia in the absence of
hypertension and/or proteinuria does occur.
The antenatal onset of pre-eclampsia and eclampsia is, by definition, after the 20th week of
pregnancy. Most cases of eclampsia present in the third trimester of pregnancy, with about
80% of eclamptic seizures occurring intrapartum or within the first 48 hours following
delivery. Rare cases have been reported prior to 20 weeks' gestation or as late as 23 days’
postpartum. Other than early detection of pre-eclampsia, no reliable test or symptom
complex predicts the development of eclampsia.
Mild pre-eclampsia may progress to severe pre-eclampsia and eclampsia very suddenly with
little or no warning. In addition, women with pre-eclampsia do not feel ill until the condition
is severe and the disease is life threatening. Early detection by regular antenatal monitoring
and careful follow-up of those with mild pre-eclampsia is therefore essential for the early
diagnosis and treatment of severe pre-eclampsia.
Definition
The definition of hypertension during pregnancy has changed over the years:
In the past it has been recommended that an incremental increase of 30 mm Hg systolic
or 15 mm Hg diastolic blood pressure be used as a diagnostic criterion, even when
absolute values remain <140/90 mm Hg
More recently, the diagnostic criterion for gestational hypertension is based on a diastolic
blood pressure reading of 90mmHg or more
Some use mean arterial pressure (MAP) of 90mmHg or more in the second trimester. MAP
appears to be a better predictor for pre-eclampsia than systolic BP (sBP), diastolic BP
(dBP), or increased BP (Cnossen et al, 2008). MAP can be calculated as follows:
MAP ≈ dBP + [1/3 (sBP-dBP)]
or equivalently
MAP ≈ 2/3(dBP) + 1/3 (sBP)
Blood pressure measurements in the first and second trimester at the first
Blood
pressure
measurements
for with
healthy
normotensive
womendo
innot
thehelp
first
antenatal
visit for
healthy women
normal
blood pressures
12
and
second
trimester
do
not
help
predict
pre-eclampsia.
???Ref
predict pre-eclampsia (Hofmeyr and Belfort, 2009).
The diagnostic criteria for pre-eclampsia are diastolic blood pressure reading of 90mmHg
or more, with proteinuria (greater than 1+), after 20 weeks gestation.
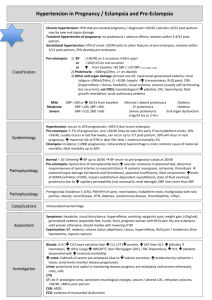
Table XX provides an overview of diagnostic criteria for the different hypertensive disorders
in pregnancy.
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
11
Table XX. Differential diagnosis of hypertensive disorders in pregnancy
Diagnosis
Chronic hypertension
Diagnostic criteria
Diastolic BP 90 mm Hg or more prior to the first 20
weeks of gestation
Women with chronic hypertension
Any of the following are seen after 20 weeks’
gestation:
Pre-eclampsia superimposed on
chronic hypertension
- New or worsening proteinuria
- Sudden increase in BP in a woman whose
hypertension has previously been well controlled
- One or more adverse conditions associated with
pre-eclampsia and/or eclampsia
Two readings of diastolic BP 90 mm Hg or more but
below 110 mm Hg 4 hours apart after 20 weeks
gestation, no proteinuria.
Postpartum:
Gestational hypertension
- Transient hypertension of pregnancy if preeclampsia is not present at the time of delivery and
blood pressure returns to normal by 12 weeks
postpartum (a retrospective diagnosis) or
- Chronic hypertension if the elevation persists
beyond 12 weeks postpartum.
Mild pre-eclampsia
Two readings of dBP 90 mm Hg or more but below
110 mm Hg 4 hours apart
Proteinuria up to 2+
Severe pre-eclampsia
dBP 110 mm Hg or more
Proteinuria 3+ or more
A pregnant woman or a woman who has recently
given birth is found unconscious or having convulsions
(fits)
Eclampsia
dBP 110 mm Hg or more
Proteinuria 2+ or more
dBP = diastolic BP
sBP = systolic BP
Screening
Hypertensive disorders in pregnancy are a major contributor to maternal mortality
worldwide. With standard refocused antenatal care, providers can detect blood pressure
elevation and the presence of proteinuria, ensure initiation of appropriate management
at the appropriate level of care, and prevent many of these deaths. Improved detection and
care should lead to a better outcome. If a woman develops hypertension, and/or
proteinuria, providers will need to closely monitor her and encourage her to give birth in a
health facility with skilled birth attendants.
Edema of the feet and lower extremities is not
considered a reliable sign of pre-eclampsia.
12
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
Reference manual
Detecting proteinuria
The presence of proteinuria changes the diagnosis from gestational hypertension to preeclampsia. Detection of proteinuria is therefore key for making a diagnosis of pre-eclampsia.
Once pre-eclampsia has been diagnosed, it would require a considerable increase in
surveillance, often including admission. The social and financial repercussions of this for the
woman and the economic consequences for the healthcare system are considerable. It is
therefore important that tests for proteinuria are accurate.
Although proteinuria is most commonly associated with pre-eclampsia or eclampsia, a
woman's urine can test positive for protein if she is severely anemic, has kidney disease, or
has a UTI, or if the urine has been contaminated by blood (or if she has schistosomiasis),
vaginal discharge, or amniotic fluid. Urine tested for protein should therefore be a clean
catch, midstream specimen of urine. Vaginal secretions and discharge are common in
pregnancy and, if mixed with urine, give a positive test for protein. To avoid this, it is
important that:
-
The vulva is cleaned with water
-
The labia minora are spread
-
While urine is being passed, the middle part of the stream is caught in a clean
container.
Methods for measuring proteinuria differ from country to country and may vary by type of
resources available at the facility. Methods to evaluate proteinuria include:
Quantitation of a timed collection: This has been the gold standard for many decades
and is expressed as the amount of protein excreted in the urine per unit time. Twenty
four-hour specimens have been traditionally used, but more recently 12-hour collections
(and even 2-hour collections) have been validated (Hofmeyr and Belfort, 2009).
Urinary protein:creatinine ratio: This is used in some institutions instead of a timed
protein collection. A review conducted by Côté et al showed that the spot
protein:creatinine ratio is a reasonable “rule-out” test for proteinuria of 0.3 g/day or
more, among otherwise healthy women with gestational hypertension with or without
proteinuria on dipstick. However, they did not advocate use of the spot protein:creatinine
ratio or spot albumin:creatinine ratio for monitoring or quantifying proteinuria in
pregnancy (Côté et al, 2008).
Urine dipsticks: Urinalysis by visual reagent strip tests is widely performed in antenatal
clinics and in the community by various health professionals. A review by Waugh et al
(2004) showed that significant proteinuria, with point-of-care urine dipstick analysis,
cannot be accurately detected or excluded at the 1+ threshold and is not recommended
for diagnosing pre-eclampsia.
Dipstick method:
-
The end of the stick is dipped into the urine and excess shaken off by tapping the
stick on the side of the container
-
The result is then read by comparison with the color chart on the label at the
time indicated on the dipstick container.
Boiling urine: Boiling urine was widely used before dipsticks were widely available. Some
facilities may still use this method to test for protein in the urine.
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
13
Boiling method:
-
Boil the top half of the urine in a test tube
-
Compare the top half of the urine with the unboiled bottom half (the boiled part
may become cloudy)
-
Add 1–2 drops of 2–3% acetic acid. Do this even if the urine has not become
cloudy
-
If, after adding the acetic acid, the boiled part of the urine remains cloudy,
protein is present in the urine
-
If the boiled urine was not cloudy to begin with, but becomes cloudy when acetic
acid is added, this is another indication that protein is present
-
If cloudy urine becomes clear when acetic acid is added, protein is not present.
While the measure of proteinuria is a poor predictor of either maternal or fetal complications
in women with pre-eclampsia (Thangaratinam et al, 2009), it remains one of the criteria for
the diagnosis of pre-eclampsia. Further work is needed to compare the different methods to
evaluate proteinuria in the management of pregnant women with hypertension, particularly
with respect to accuracy of diagnosis and the effect on inappropriate admissions and
discharges.
REMEMBER:
Because of changes in metabolism during pregnancy, the pregnant woman will spill
some protein in her urine and this is normal, as long as it does not exceed 1+.
Although proteinuria is most commonly associated with pre-eclampsia or eclampsia, a
woman's urine can test positive for protein if she is severely anemic, has kidney
disease, or has a UTI, or if the urine has been contaminated by blood (or if she has
schistosomiasis), vaginal discharge, or amniotic fluid.
The woman may have pre-eclampsia/eclampsia AND other conditions that may cause
proteinuria.
Detecting hypertension
BP readings are prone to inaccuracy due not only to observer and device error, but also to
variability of blood pressure and to rise in BP caused by anxiety/fear due to the effects of
attendance at the clinic (white-coat hypertension) (Higgins and de Swiet, 2001).
Simple measures to reduce observer error when taking BP measurements:
14
Choose the correct cuff size.
-
The cuff must be at least 2–3 cm (1 inch) above the elbow and should encircle at
least three–fourths of the circumference of the arm; otherwise, a false high
reading will be obtained.
-
The length of the bladder on the device should be 80 percent of the
circumference of the upper arm. This means that heavy or very muscular people
with thick arms need a larger bladder.
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
Reference manual
Figure xx. Bladder on the BP device
Wrap the cuff firmly around the upper arm.
Do not kink or twist the tube on the cuff. Make sure that the stethoscope fits into
your ears firmly and snugly.
If a mercury blood pressure machine is used, it must be in the vertical position, and
your eyes must be approximately at the same level as the top of the mercury column
or the reading will not be accurate.
If an aneroid manometer is used, place the manometer in your direct line of sight.
Systolic blood pressure is taken at the point at which the arterial sound appears.
Diastolic blood pressure is taken at the point at which the arterial sound disappears.
Simple measures to reduce device error when taking BP measurements:
Measurements should be made with a mercury sphygmomanometer; in centers
where the use of mercury has been banned for clinical purposes, the mercury
sphygmomanometer will have to be replaced by an electronic device that has been
validated for pregnancy.
The sphygmomanometer should be regularly calibrated
Aneroid Manometer
Check that the needle
is at the zero mark at
the start and the end
of the measurement.
Figure xx. Aneroid manometer
Check to see that the screw valve on the ball works properly before using the BP
machine.
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
15
Pump up the bladder and watch for any air leaks. If the mercury column or aneroid
needle does not rise steadily as the ball is pumped, suspect a leak.
Simple measures to reduce variability of BP measurements:
Remove all tight clothes from around the arm. Tight clothes may partially block the
artery and give a false low reading.
The woman should not smoke or drink alcohol or coffee within 15 minutes of a blood
pressure measurement.
Make sure that the woman is as relaxed and comfortable as possible.
It is better if she rests for several minutes in that position before the measurement.
Ask her not to talk during the measurement.
Blood pressure should always be checked in the sitting position (BP will be highest in
the sitting position, somewhat lower when she lies supine, and lowest when she lies
on her side). The elbow (brachial artery) should be at the level of the heart.
-
She should sit with her back supported and her elbow at about the level of her
heart with her arm supported.
-
Her legs should not be dangling. Her feet should be supported or on the ground.
If the woman cannot sit, take the BP with her lying tilted to the left side.
-
Lying on the back is not a good position because the weight of the gravid uterus
exerts pressure on the inferior vena cava, thereby causing a drop in blood
pressure.
-
When BP is checked with the woman in left lateral recumbent, the superior arm
has 10-12 mmHg lower BP than the inferior arm
REMEMBER:
The normal BP is between 80/60 mmHg and 140/90 mmHg
When the pre-pregnancy BP is not known, the BP taken before 20
weeks is considered the woman's normal BP
Because of changes in cardiac output and blood volume, hormonal
changes which mediate a decrease in peripheral vascular resistance,
smooth muscle-relaxing effect of progesterone, and heat production by
the foetus, the BP will have some normal variations during pregnancy:
- Systolic and diastolic BP begin to fall in the 1st trimester, decreasing
until mid-pregnancy and gradually return to non-pregnancy baseline
by term
- There is a slight fall in systolic and considerable decrease in diastolic
later in gestation
Detecting hypertensive disorders in pregnancy
At every antenatal and postnatal visit:
1. Take a targeted symptom history. ASK the woman if she has had any of these
symptoms: epigastric pain (heartburn), headaches, or visual problems (double vision,
partial vision, rings around lights). Note that these are the danger signs the pregnant /
postpartum woman should herself be aware of and watch for.
2. Take the blood pressure at every visit.
16
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
Reference manual
If the diastolic BP is >90 mmHg, check that the BP cuff is the right size and that the
BP machine is functioning properly.
If there are no problems with the BP cuff size and machine, have the woman lie on
her left side for 20 minutes, then recheck it again with her sitting up
–
If the blood pressure is normal, educate the woman about danger signs and have
her return in two weeks for a BP check
–
If the blood pressure is still elevated, check a midstream urine sample for protein
and plan to check the BP again in 4 hours.
–
If the BP is still elevated 4 hours after the first reading, this is considered
hypertension.
3. Take a good personal and family history of:
Epilepsy
Hypertension
Renal or heart disease
Cerebro-vascular accident (CVA)
4. Check urine for protein at all visits after 20 weeks of pregnancy. Proteinuria is defined as
the presence of 300 mg or more of protein per liter in a clean catch, midstream
specimen of urine. Usually proteinuria follows a rise in blood pressure, but occasionally
it is the first sign of the disease.
If there is greater than 1+ protein in the urine:
–
Verify that the sample was a mid-stream/clean-catch sample
–
Check for sexually transmitted infections (STI) (especially if the woman has
abundant vaginal secretions). If the woman has an STI, treat according to cause
and following protocols.
–
Blood or white blood cells in the urine may cause a false positive test for
proteinuria because both blood and white blood cells are proteins. Rule out a
urinary tract infection, schistosomiasis (in endemic areas), and kidney infections.
If the woman has an UTI, schistosomiasis, or kidney infection, treat according to
cause and following protocols.
–
Rule-out anemia: Check haemoglobin (if there is a laboratory) or check for signs
of anemia; if the woman has moderate anemia, treat appropriately and rule out
hookworm and malarial infections; if the woman has severe anemia (<7 gm/dL),
refer her to a hospital/doctor.
–
If the woman’s blood pressure is elevated and she has protein in her urine
(>1+), check the biceps and/or patellar reflexes.
5. Test the woman’s biceps and/or patellar reflexes if the woman’s blood pressure is
elevated and she has protein in her urine (>1+).
Testing reflexes is part of an examination of the nervous system. It is very helpful
for midwives to know how to test a few basic reflexes on adults. Hyper-reflexia can
indicate many diseases of the nervous system or edema of the brain (cerebrum) in a
pregnant woman. A woman with cerebral edema is very likely to develop
eclampsia (convulsions).
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
17
Using a Reflex Hammer
A reflex hammer is used to check the deep tendon reflexes. Once you are
experienced, you may be able to use your fingers, the side of your hand, your
knuckles, or the head of a stethoscope instead. For beginners (learners), it may be
helpful to use a reflex (percussion) hammer.
1. Hold the hammer loosely between your thumb and index finger.
2. Bring the hammer down onto the tendon in a rapid, smooth movement.
3. Tap quickly and firmly.
4. Lift the hammer back up quickly.
5. Watch for how fast the response is. It is the speed of the response, not how
far the limb moves, that tells you if her reflexes are normal.
Reflexes are usually given a grade of 0 to +4. The scale of grading is:
0
+1
no response
low but within normal response
+2
average or normal response
+3
brisker than average
+4
very brisk, hyperactive, abnormal, may have rhythmic tremors (clonus)
When checking reflexes, always check both sides (both arms or both legs). Check
that the response is similar on both sides. The biceps and patellar reflexes are the
common ones to use when looking for pre-eclampsia in pregnant women.
Biceps Reflex
1. Bend the woman's arm about halfway.
2. With your fingers, feel for her tendon on the inside of her elbow (antecubital
fossa). If it is difficult to locate, move her arm up and down while feeling. You will
notice a cord-like tendon.
3. If the woman is lying down, the bed will support her arm. If she is sitting up, you
will need to support her arm on yours. Place your thumb on the tendon.
Figure XX. Biceps Reflex Testing
Adapted from: Marshall, M.A., Buffington, S.T. Life-saving skills manual for midwives. 3rd
edition. Washington, DC: American College of Nurse Midwives (ACNM), 1998
18
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
Reference manual
4. Strike your thumbnail, which is positioned over the tendon. This causes the
biceps muscle to contract. You may or may not see the slight contraction at the
woman's elbow.
5. You will be able to feel the response from the tendon through your thumb. You
can grade the response by how fast you are able to feel the reflex response. You
will need to check many reflexes before you develop an awareness of what is
normal. Check your family, friends, and all of your clients to gain experience.
Patellar Reflex
1. Have the woman sit on the examining table or couch. Her legs should hang
freely.
2. Feel for her tendon right below the kneecap (patella). If it is difficult to locate,
move her lower leg a little while feeling at the same time.
Figure XX. Patellar Reflex Testing, seated patients
Adapted from: Marshall, M.A., Buffington, S.T. Life-saving skills manual for midwives. 3rd
edition. Washington, DC: American College of Nurse Midwives (ACNM), 1998
3. Strike the tendon with a quick, firm tap and lift up immediately. You may also
use the side of your hand or your knuckle to tap the tendon.
4. Tapping the tendon will cause the quadriceps muscle to contract, causing the
lower leg to move.
5. The patellar reflex can also be tested with the woman lying in bed. Place one
hand under the leg, supporting it, and tap.
6. If the woman is tense and contracting her muscles, you will not get an accurate
test of her reflexes. You may need to talk to her and keep her attention away
from what you are doing.
If the reflexes are brisk (+3 or +4), refer her to a hospital/doctor.
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
19
Remember:
20
A woman with severe pre-eclampsia who has hyper-reflexia (+3 or +4) is very ill.
She must be properly stabilized and transferred to a doctor/Level 3 facility as
quickly as possible.
Care of women with pre-eclampsia can save the lives of both the woman and
fetus.
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
Reference manual
Prevention of pre-eclampsia and/or eclampsia
Primary prevention of pre-eclampsia
By definition, primary prevention can help to avoid the development of a disease. Primary
prevention is difficult to achieve for pre-eclampsia because the cause is not well understood
and most factors associated with it are difficult to avoid or manipulate. Nevertheless, there
are certain interventions that can serve to prevent pre-eclampsia.
Prevention of too early and too late pregnancies with family planning
Pre-eclampsia is unique to pregnancy, and adolescent and older (more than 35 years of
age) women are at higher risk of pre-eclampsia, therefore prevention of high risk
pregnancies will prevent pre-eclampsia (Dekker and Sibai, 2001). Of course, women will
want to have children and cannot indefinitely wait for pregnancy. However, family planning
can help to delay the first pregnancy until the woman is at least 20 or 21 years of age; can
limit pregnancies beyond 35 years of age if women no longer want to continue having
children; and can ensure adequate spacing between pregnancies to allow the woman to
recover between pregnancies.
Prevention and/or treatment of obesity
Obesity is a definite risk for developing gestational hypertensive disorders, including preeclampsia. Obesity has a strong link with insulin resistance. The exact mechanisms by which
obesity/insulin resistance are associated with an increased risk for pre-eclampsia are not
completely understood. Prevention of or effective treatment of obesity, or both, could result
in a substantial decrease in its frequency.
Overweight and obese women should have counseling before pregnancy so that they are
aware of the risks and can try to modify their weight before pregnancy. Once an overweight
or obese woman is pregnant, she should eat a healthy, well-balanced, and monitored diet,
and try to limit weight gain to 7-11.5 kg (for BMI 25-29.9 kg/m2) or 5-9 kg (for BMI ≥ 30
kg/m2) (Rasmussen et al, 2009).
Prevention of IUGR
Pre-eclampsia increases the risk for intrauterine growth restriction (IUGR). Having a low
birthweight as a consequence of IUGR has also been identified as an important risk factor of
the so-called insulin resistance syndrome in adult life. Prevention of pre-eclampsia and IUGR
could therefore, at least theoretically, contribute to primary prevention of pre-eclampsia
(and IUGR) in the next generation (Dekker and Sibai, 2001).
Smoking
Cigarette smoking is associated with a 30–40% decrease in the risk of pre-eclampsia
(Conde-Agudela and Belizan, 2000). However, this benefit is cancelled out by the
substantial negative effects of smoking on fetal growth, risk for placental abruption, and
general health. Understanding the mechanisms of the preventive effects of smoking on preeclampsia could help to unravel important aspects of its pathophysiology.
Reducing risks related to paternity
While findings from studies on sperm exposure and paternity suggest an association
between length of exposure to sperm and the man’s history of fathering a pre-eclamptic
pregnancy, it is difficult to recommend preventive interventions for these two risk factors.
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
21
However, health-care providers should realize that the antenatal care of a multiparous
patient with a new partner should be the same as in a woman presenting with her first
pregnancy, at least as far as the risk for pre-eclampsia is concerned (Robillard et al, 1999;
Roberts et al, 2001)
Use of low-dose aspirin to prevent pre-eclampsia
The use of low-dose aspirin during pregnancy decreases the risk of pre-eclampsia for
women considered at increased risk (Vogel et al, 2009), and also decreases rates of preterm birth, perinatal death, and incidence of small-for-gestational age infants (Gauer and
Atlas, 2008). There is no evidence of harm from low-dose aspirin therapy—including
placental abruption, antenatal admissions, fetal intraventricular hemorrhage and other
neonatal bleeding complications, admission to neonatal care unit, induction of labor, or
caesarean delivery—regardless of the woman’s risk status (Coomaraswamy et al, 2003).
The aspirin dosage used in studies ranged from 50 mg/day to 150 mg/day. Villar et al
showed a greater effect among women treated with doses greater than 75 mg/day of
aspirin (Villar et al, 2004).
Calcium supplementation to prevent pre-eclampsia
In a Cochrane review (Hofmeyr et al, 2009), calcium supplementation was associated with
reduced hypertension and pre-eclampsia, particularly for those at high risk of the disease
and with a low baseline dietary calcium intake (for those with an adequate calcium intake
the difference was not significant). No side effects of calcium supplementation were
recorded in the trials reviewed.
The data lend support to calcium supplementation for women at high risk of pre-eclampsia
and in communities with low dietary calcium intake. The absence of convincing evidence of
effectiveness from the largest trial (n=4589), which recorded no reduction in the rate or
severity of pre-eclampsia or in the timing of onset, have discouraged the use of calcium
supplementation in high resource countries.
Diet and exercise
Providers frequently advise women to make a range of changes to their diet and lifestyle to
reduce their risk of developing pre-eclampsia. The following interventions have been
evaluated in randomized trials: (1) aerobic exercise (Kramer, 2003), (2) protein
restriction(Kramer, 2003), (3) protein supplementation (Kramer, 2003), and (4) increasing
or decreasing salt intake (Duley et al, 2003). Because the studies were small, there is
insufficient evidence to either recommend or counsel against their use.
Other dietary supplements to prevent pre-eclampsia
Prophylactic magnesium (Sibai et al, 2005; Makrides and Crowther, 2003) and zinc
(Mahomed, 2003) supplementation have not been shown to be beneficial in preventing preeclampsia. Three randomized trials of fish oil supplementation for women at high risk for
pre-eclampsia revealed no reduction in the incidence of pre-eclampsia (Makrides et al,
2003). A recent study showing the benefits of vitamins C and E to prevent pre-eclampsia
was encouraging but needs further confirmation (Conde-Agudela and Belizan, 2000).
Secondary prevention (screening and detection)
Secondary prevention activities are aimed at early disease detection, thereby increasing
opportunities for interventions to prevent progression of pre-eclampsia. The ability to
prevent eclampsia is limited by lack of knowledge of its underlying cause. Prevention has
focused on identifying women with elevated blood pressure and/or proteinuria, followed by
close clinical and laboratory monitoring to recognize disease progression. Although these
22
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
Reference manual
measures do not prevent pre-eclampsia, they may be helpful in preventing some adverse
maternal and fetal sequelae associated with symptoms and in preventing progression to
eclampsia.
Focused antenatal care
Focused antenatal care is the most important part of secondary and tertiary prevention. The
decrease in maternal mortality and serious morbidity results mainly from the screening
(checking BP and testing urine for protein) and tertiary prevention (such as timed delivery)
associated with organized antenatal care. In order for antenatal care to be effective,
however, health care providers must be adequately trained to identify, prevent, and
manage pre-eclampsia and should have all of the essential equipment (in particular
accurate sphygmomanometers and means to detect protein in the urine), commodities, and
consumables (in particular for testing urine). In addition, adequate systems must be in
place to stabilize the woman and transfer her to the appropriate level of care.
Women, families, and communities need to understand danger signs and the importance of
seeking early and regular antenatal care. During antenatal care, health care providers can
assist women and their families to develop a birth preparedness and complication readiness
plan that will ensure that women access care in a timely manner.
Tertiary prevention (management of severe pre-eclampsia
and eclampsia)
Tertiary prevention focuses on the prevention of complications in women with preeclampsia. Reduction of maternal and fetal/newborn mortality and serious morbidity
depends on timely diagnosis and early referral. The three major interventions for
management of severe pre-eclampsia and eclampsia are: anti-convulsant therapy, antihypertensive treatment, and timed delivery of the baby.
Anti-convulsant medications
Anticonvulsant drugs are used in the management of severe pre-eclampsia to prevent the
occurrence of eclamptic fits. Trials have compared magnesium sulfate with phenytoin,
nimodipine, and diazepam for prevention of eclampsia in women with pre-eclampsia. These
studies all support magnesium sulfate as the anticonvulsant of choice for women with preeclampsia (Duley, Gulmezoglu and Henderson-Smart, 2003).
Anticonvulsant drugs are used in the management of eclampsia to control and prevent the
recurrence of eclamptic fits. Magnesium sulfate has been compared with diazepam,
phenytoin, and lytic cocktail in randomized trials.
Magnesium sulfate vs. diazepam (Duley and Henderson-Smart, 2003):
When compared to diazepam, magnesium sulfate was associated with a reduction in
the risk of maternal death and in the risk of recurrence of convulsions.
When compared to diazepam, magnesium sulfate was associated with a reduction in
the risk of an Apgar score <7 at 5 min and in length of stay in a special care baby
unit (SCBU) >7 days.
Magnesium sulfate vs. phenytoin (Duley and Henderson-Smart, 2003):
Magnesium sulfate was associated with a reduction in the relative risk of recurrent
convulsions, the risk of pneumonia, the need for ventilation, and admission to an
intensive care unit
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
23
Babies whose mothers were allocated magnesium sulphate, rather than phenytoin,
had fewer admissions to a special care baby unit and fewer died or were in SCBU for
>7 days.
Magnesium sulfate vs. lytic cocktail (usually a mixture of chlorpromazine, promethazine
and pethidine) (Duley and Gulmezoglu, 2003):
Magnesium sulfate was substantially better at preventing further fits than lytic
cocktail and there was also a non-significant trend to fewer maternal deaths with
magnesium sulfate rather than lytic cocktail.
There was also a non-significant trend to fewer baby deaths (stillbirths and neonatal
deaths) in babies whose mothers were allocated magnesium sulfate, rather than lytic
cocktail.
Magnesium sulfate reduces the risk of eclampsia (Magpie Trail Collaborative Group, 2002)
without any substantive effect on longer-term morbidity and mortality for the women or
children (Magpie Trail Collaborative Group, 2007). Data from the Magpie (magnesium
sulfate for prevention of eclampsia) Trial (Smyth et al, 2009) provide reassurance about the
longer-term safety of magnesium sulfate when used for women with pre-eclampsia. There
appears to be no substantive effect on women's subsequent fertility, or their use of health
care services in the two years after the birth. Data from the main Magpie Trial follow up
study demonstrated that magnesium sulfate has no clear effect on the child's risk of severe
neuro-developmental delay (Magpie Trail Collaborative Group, 2007).
For prevention of occurrence and recurrence of eclamptic convulsions/fits in women with
severe pre-eclampsia and eclampsia, magnesium sulfate is more effective and has
fewer risks than diazepam, phenytoin, and lytic cocktail.
Do NOT give lytic cocktail, phenobarbital, or phenytoin
(Dilantin) to pregnant women.
Anti-hypertensive medications
An important objective in the care of a woman with severe hypertension, with or without
proteinuria, is to reduce blood pressure in order to avoid hypertensive encephalopathy and
cerebral hemorrhage. For this reason, the aim in treating severely hypertensive women is to
keep the blood pressure below dangerous levels (dBP<110 mmHg). It remains unclear
whether antihypertensive drug therapy for mild to moderate hypertension during pregnancy
is worthwhile (Duley, 2003).
While the goal of treatment of hypertension in pregnancy is to reduce maternal risk, careful
attention must be made not to harm the fetus. Antihypertensive medications may permit
prolongation of the pregnancy and thereby improve fetal maturity, but administration of a
powerful vasodilator will result in a decreased intervillous blood flow. Acute falls in maternal
systemic blood pressure may thus result in fetal compromise.
If antihypertensive treatment is required, there is no clear choice of drugs (Duley, 2003;
Cnossen et al, 2008). In general, however: (1) labetolol, nifedipine, and hydralazine are the
anti-hypertensive medications most commonly recommended for management of
hypertensive disorders in pregnancy; and (2) Diuretics (furosemide, hydrochlorothiazide)
and angiotensin-converting enzyme inhibitors (captopril) are contraindicated for use as antihypertensive agents during pregnancy. Refer to Table XX for a broad overview of
advantages and disadvantages of anti-hypertensive medications that may be used for
management of pre-eclampsia/eclampsia (Cnossen et al, 2008; National High Blood
Pressure Education Program Working Group on High Blood Pressure in Pregnancy, 2001).
24
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
Reference manual
Table xx. Advantages and disadvantages of selected anti-hypertensives for use during
pregnancy
Medication
Calcium channel
blockers (nifedipine)
Combined alpha and
beta-blocker
(labetalol
hydrochloride)
Advantages
A multicenter prospective cohort
study of first-trimester drug
exposures reported no increase
in major teratogenicity from
these agents
Causes dilatation of small
arteries
None of these agents has been
associated with any consistent ill
effects
Less likely to reduce uteroplacental perfusion than betablockers and may improve
cerebral circulation
Available data suggest that the
antihypertensive effect is not
associated with compromised
renal or uterine blood flow
Disadvantages
Experience with calcium antagonists is
limited
Safety record of labetalol in pregnancy
is not as well established as that of
methyldopa
Direct acting
vasodilator
(hydralazine)
Smooth muscle relaxant,
causing vasodilatation
Associated with more maternal and
perinatal adverse effects than other
drugs*
Alpha-Adrenergic
Agonist
(methyldopa)
Stable uteroplacental blood flow
and fetal hemodynamics
No long-term adverse effects on
development among children
exposed to methyldopa in utero
Causes somnolence in many individuals
β -blockers
(atenolol)
None of these agents has been
associated with any consistent ill
effects
β blockers prescribed during early
pregnancy, specifically atenolol, may be
associated with growth restriction
Long-term follow-up studies are lacking
Diuretics
(furosemide,
hydrochlorothiazide)
Reduce blood pressure
Reduce maternal plasma volume and
can cause electrolyte disturbances
Angiotensinconverting enzyme
inhibitors (captopril)
May be safe in early pregnancy
but women taking these agents
should be warned about the
possible risks of this class of
drugs in pregnancy and advised
to discontinue
Administration during the second and
third trimesters can result in a number
of fetal adverse effects, including
growth retardation, renal failure,
persistent patent ductus arteriosus,
respiratory distress syndrome, fetal
hypotensive syndrome, and prepartum
death
Induction of labor
Apart from Cesarean operation or induction of labor (and therefore delivery of the placenta),
there is no known cure for pre-eclampsia. A decision to induce labor will need to weigh
benefits and risks for both the woman and fetus. The National High Blood Pressure
* Recent data on maternal and perinatal adverse effects associated with hydralazine have led some
researchers to state that hydralazine should no longer be considered the anti-hypertensive medication
of choice (Smyth et al, 2009).
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
25
Education Program Working Group on High Blood Pressure in Pregnancy recommends the
following when considering delivering the baby to manage gestational hypertension:
“First, any therapy for pre-eclampsia other than delivery must have as its
successful end point the reduction of perinatal morbidity and mortality.
Second, the cornerstone of obstetric management of pre-eclampsia is based on
whether the fetus is more likely to survive without significant neonatal complications
in utero or in the nursery” (National High Blood Pressure Education Program Working
Group on High Blood Pressure in Pregnancy, 2001).
The decision to terminate pregnancy will depend upon:
1. Severity of the disease
2. Gestational age
3. Maternal and fetal condition
The WHO (WHO, 2003) recommends the following for timing of delivery:
In severe pre-eclampsia, delivery should occur within 24 hours of the onset of
symptoms.
In eclampsia, delivery should occur within 12 hours of the onset of convulsions/fits.
When the gestational hypertension is mild, induction of labor is associated with improved
maternal outcome and should be advised for women beyond 37 weeks’ gestation
(Koopmans et al, 2009).
26
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
Reference manual
Overview of interventions to prevent pre-eclampsia and
eclampsia
For an overview of preventive interventions, see Table XX.
Table xx. Overview of interventions to prevent pre-eclampsia and eclampsia
Prevention
Intervention
Pregnancy outcome
Prevention of IUGR
Theoretically contributes to
primary prevention of preeclampsia (and IUGR) in the
next generation
Recommended
Family planning
Potential to reduce
pregnancies at risk for preeclampsia
Recommended
Pre-conceptual
prevention and/or
treatment of obesity
Potential to reduce preeclampsia
Recommended
Smoking
Reduces risk of preeclampsia
Not recommended
Low-dose aspirin
Reduces pre-eclampsia
Reduces fetal or neonatal
deaths
Advise women with more
than one moderate risk
factor for pre-eclampsia to
take 75 mg of aspirin daily
from 12 weeks gestation
until the birth of the baby.
Calcium
supplementation
Reduces pre-eclampsia in
those at high risk and with
low baseline dietary calcium
intake
No effect on perinatal
outcome
Primary
Magnesium or zinc
supplementation
Fish oil
supplementation and
other sources of
fatty acids
Heparin or lowmolecular weight
heparin
Anti-oxidant vitamins
(C, E)
Secondary
Recommendation
BP and urinary
protein screening
during antenatal and
postnatal visits
No reduction in preeclampsia
Advise women at risk of
gestational hypertension
living in communities with
low dietary calcium intake,
to take 1 G of calcium daily
from 12 weeks gestation
until the birth of the baby.
Insufficient evidence to
recommend*
No effect on low- or high-risk
populations
Insufficient evidence to
recommend*
Reduces pre-eclampsia in
women with renal disease
and thrombophilia
Reduced pre-eclampsia in
one trial
No reduction in preeclampsia
Reduces some adverse
maternal and fetal sequelae
Assists in preventing
progression to eclampsia
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
Insufficient evidence to
recommend*
Insufficient evidence to
recommend*
Recommend for all pregnant
women
27
Prevention
Intervention
Protein or salt
restriction
Anti-convulsive drugs
Magnesium
sulfate
Diazepam
Tertiary
Anti-hypertensive
drugs
Induction of labor
Pregnancy outcome
No reduction in preeclampsia
Recommendation
Insufficient evidence to
recommend*
Reduces the risk of
eclampsia without any
substantive effect on longerterm morbidity and mortality
for the women or children
Recommend for women with
severe pre-eclampsia and
eclampsia
When compared to
diazepam, magnesium
sulfate was associated with a
reduction in the risk of
maternal death and in the
risk of recurrence of
convulsions.
When compared to
diazepam, magnesium
sulfate was associated with a
reduction in the risk of an
Apgar score <7 at 5 min and
in length of stay in a special
care baby unit (SCBU) >7
days.
Improves maternal outcome.
May permit prolongation of
the pregnancy and thereby
improve fetal maturity.
Acute falls in maternal
systemic blood pressure can
result in fetal compromise.
Improves maternal and fetal
outcome when carried out
according to
recommendations for severe
pre-eclampsia and eclampsia
Recommend if magnesium
sulfate is not available
Recommend if diastolic BP
110 mm Hg or more
Consider for women beyond
37 weeks’ gestation with
mild pre-eclampsia.
Recommend based on
severity of the disease,
gestational age, and
maternal and fetal condition
* Insufficient evidence=small trials or inconclusive results
28
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
Reference manual
Management of pre-eclampsia and eclampsia
Management protocols are copied from: WHO. MCPC. Geneva: WHO, 2003.
Introduction
In order to detect early signs of pregnancy-induced hypertension and pre-eclampsia, regular
antenatal visits are necessary, especially in the third trimester of pregnancy. At each
antenatal visit, the woman’s blood pressure must be measured and her urine should be
checked for protein from 20 weeks gestation and/or if diastolic blood pressure is more than
90 mmHg. Pregnant women should be encouraged to come for antenatal care early in their
pregnancy so that a baseline value for their blood pressure can be obtained.
Survival and positive outcomes for the woman and her baby depend on timely diagnosis and
timely treatment at the appropriate level of care. In general:
Women with gestational hypertension whose diastolic BP is less than 90 mmHg can
be managed at an outpatient clinic with basic emergency obstetric care facilities
Women with mild pre-eclampsia (<37 weeks gestation) can be managed at an
outpatient clinic with basic emergency obstetric care facilities
Women with mild pre-eclampsia (>37 weeks gestation) should be managed at a
facility with comprehensive emergency obstetric care facilities
Women with severe pre-eclampsia should be managed at a facility with
comprehensive emergency obstetric care facilities
Women with eclampsia should be managed at a facility with comprehensive
emergency obstetric care facilities
If there is a rise in blood pressure, the woman should be closely monitored at frequent
intervals. If proteinuria develops, she should receive care in a health facility capable of
coping with a woman who may develop severe pre-eclampsia or eclampsia.
Gestational hypertension
Diagnostic criteria
Two readings of diastolic BP 90 mm Hg or more but below 110 mm Hg 4 hours apart after
20 weeks gestation
No proteinuria
Management
The woman is usually managed as an outpatient and followed up weekly at home or at a
local clinic. Management on an outpatient basis at each visit:
Monitor blood pressure, urine (for proteinuria) and fetal condition (growth, movement,
heart rate) weekly
Check if the woman has severe headache, visual disturbances or abdominal pain
Counsel the woman and her family about the danger signals of severe pre-eclampsia,
ensuring that they know the importance of obtaining immediate medical help if any of the
signs develop.
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
29
If the blood pressure decreases to normal levels and there are no other complications, the
condition has stabilized and the woman should be allowed to proceed with normal labour
and childbirth.
Refer for care at a health care facility capable of coping with a woman who may develop
severe pre-eclampsia or eclampsia if:
o
the blood pressure rises
o
proteinuria develops
o
there is significant fetal growth restriction (signs of poor fetal growth)
o
there is evidence of fetal compromise (abnormal fetal movements, heart rate,
or growth)
Mild pre-eclampsia - Gestation less than 37 weeks
Diagnostic criteria
Two readings of diastolic BP 90 mm Hg or more but below 110 mm Hg 4 hours apart after
20 weeks gestation
Proteinuria up to 2+
Management
If signs remain unchanged or normalize, follow up twice a week as an outpatient:
Monitor blood pressure, urine (for proteinuria), reflexes and fetal condition.
Counsel the woman and her family about danger signals of severe pre-eclampsia or
eclampsia.
Encourage additional periods of rest.
Encourage the woman to eat a normal diet (salt restriction should be discouraged).
Do not give anticonvulsants, anti-hypertensives, sedatives or tranquillizers.
If follow-up as an outpatient is not possible, admit the woman to the hospital:
30
-
Provide a normal diet (salt restriction should be discouraged)
-
Monitor blood pressure, reflexes and fetal condition (twice daily)
-
Monitor urine for proteinuria (daily)
-
Do not give anticonvulsants, anti-hypertensives, sedatives or tranquillizers unless
blood pressure or urinary protein level increases
-
Do not give diuretics. Diuretics are harmful and only indicated for use in preeclampsia with pulmonary edema or congestive heart failure
-
If the diastolic pressure decreases to normal levels or her condition remains
stable, send the woman home:
o
Advise her to rest and to watch out for significant swelling or symptoms of severe
pre-eclampsia
o
See her twice weekly to monitor blood pressure, urine (for proteinuria) and fetal
condition and to assess for symptoms and signs of severe pre-eclampsia
o
If diastolic pressure rises again, readmit her
o
If the signs remain unchanged, keep the woman in the hospital. Continue the
same management and monitor fetal growth by symphysis-fundal height
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
Reference manual
o
If there are signs of growth restriction, consider early delivery. If not,
continue hospitalization until term
If urinary protein level increases, manage as severe pre-eclampsia (see below)
Note: Symptoms and signs of pre-eclampsia do not completely disappear until after
pregnancy ends.
Mild pre-eclampsia - Gestation of 37 complete weeks or
more
Diagnostic criteria
Two readings of diastolic BP 90 mm Hg or more but below 110 mm Hg 4 hours apart after
20 weeks gestation
Proteinuria up to 2+
Management
Assess the cervix and expedite delivery:
-
If the cervix is favorable (soft, thin, partly dilated), rupture the membranes with
an amniotic hook or a Kocher clamp and induce labor using oxytocin or
prostaglandins.
-
If the cervix is unfavorable (firm, thick, closed), ripen the cervix using
prostaglandins or a Foley catheter or deliver by cesarean operation.
Monitor blood pressure, urine (for proteinuria), reflexes and fetal condition.
Counsel the woman and her family about danger signals of severe pre-eclampsia or
eclampsia.
Encourage additional periods of rest.
Encourage the woman to eat a normal diet (salt restriction should be discouraged).
Do not give anticonvulsants, anti-hypertensives, sedatives or tranquillizers
Severe pre-eclampsia and eclampsia
Severe pre-eclampsia and eclampsia are managed similarly with the exception that delivery
must occur within 12 hours of onset of convulsions in eclampsia. ALL cases of severe preeclampsia should be managed actively. Symptoms and signs of “impending eclampsia”
(blurred vision, hyperreflexia) are unreliable and expectant management is not
recommended.
Diagnostic criteria – severe pre-eclampsia
Diastolic BP 110 mm Hg or more
Proteinuria 3+ or more
Diagnostic criteria – eclampsia
A pregnant woman or a woman who has recently given birth is found unconscious or
having convulsions (fits), diastolic BP 110 mm Hg or more
Proteinuria 2+ or more
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
31
A small proportion of women with eclampsia have normal blood pressure.
Treat all pregnant/postpartum women with convulsions as if they have
eclampsia until another diagnosis is confirmed.
General management
If diastolic blood pressure remains above 110 mm Hg, give antihypertensive
drugs (see below). Reduce the diastolic blood pressure to less than 100 mm Hg but not
below 90 mm Hg.
Start an IV infusion and infuse IV fluids.
Give anti-convulsive drugs to prevent or treat convulsions / fits.
Catheterize the bladder to monitor urine output and proteinuria.
Never leave the woman alone. A convulsion followed by aspiration of vomit may
cause death of the woman and fetus.
Encourage the woman to lie on her side.
Assess clotting status with a bedside clotting test (see below). Failure of a clot to form
after 7 minutes or a soft clot that breaks down easily suggests coagulopathy.
1. Take 2 mL of venous blood into a small, dry, clean, plain glass test tube
(approximately 10 mm x 75 mm).
2. Hold the tube in a close fist to keep it warm (±37°C).
3. After four minutes, tip the tube slowly to see if a clot is forming. Then tip it
again every minute until the blood clots and the tube can be turned upside
down.
4. Failure of a clot to form after seven minutes or a soft clot that breaks down
easily suggests coagulopathy.
Plan to monitor the woman and fetus closely:
o
Maintain a strict fluid balance chart and monitor the amount of fluids
administered and urine output to ensure that there is no fluid overload.
o
Check BP, pulse, respirations, urinary output, reflexes and fetal heart rate hourly,
or more frequently as needed.
o
Observe color for cyanosis and need for oxygen hourly.
o
Auscultate the lung bases hourly for rales indicating pulmonary edema. If rales
are heard, withhold fluids and give furosemide 40 mg IV once.
o
Check temperature every four hours (hyperpyrexia may occur).
o
Check for signs of labor.
Anticonvulsive drugs
A key element of managing severe pre-eclampsia and eclampsia is adequate administration
of anticonvulsive drugs. Convulsions in hospitalized women are most frequently caused by
under-treatment. Magnesium sulfate is the drug of choice for preventing and
treating convulsions in severe pre-eclampsia and eclampsia. Administration is
outlined below.
32
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
Reference manual
BOX 1 Loading dose for magnesium sulfate for management of severe preeclampsia and eclampsia
Loading dose
1. Give magnesium sulfate 20% solution, 4 g IV over 5 minutes
Take one 20 mL sterile syringe
Draw 4 ampoules of MgSO4 50% (8 mL = 4 gm) into the syringe
Add 12 mL of sterile water for injection to make it 20%.
Give IV slowly over 5 minutes.
2. Follow promptly with 10 g of 50% magnesium sulfate solution, 5 g in each buttock as
deep IM injection with 1 mL of 2% Lignocaine in the same syringe.
Ensure that aseptic technique is practiced when giving magnesium sulfate deep IM
injection. Warn the woman that a feeling of warmth will be felt when magnesium
sulfate is given.
Take two 20 mL sterile syringes.
Draw 5 ampoules of MgSO4 50% (10 mL = 5 gm) into each syringe.
Add 1 mL of 2% Lignocaine in each syringe.
Give deep IM injection in each buttock.
3. If convulsions recur after 15 minutes, give 2 g magnesium sulfate (50% solution) IV
over 5 minutes.
Take one 10 mL sterile syringe
Draw 2 ampoules of MgSO4 50% (4 mL = 2 gm) into the syringe
Give IV slowly over 5 minutes.
Magnesium prevents or controls convulsions by blocking neuromuscular transmission and
decreasing the amount of acetylcholine liberated at the end plate by the motor nerve
impulse. Magnesium is said to have a depressant effect on the central nervous system. The
adverse effects of magnesium sulfate injections usually are the result of magnesium
intoxication.
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
33
As serum magnesium rises above therapeutic levels, the deep tendon reflexes are first
decreased and then disappear. At this level respiratory paralysis and/or heart block may
occur.
Magnesium acts peripherally to produce vasodilation. With low doses only flushing and
sweating occur, but larger doses cause lowering of blood pressure.
To prevent magnesium intoxication, it is important to evaluate respiratory rate, deep tendon
reflexes, and urinary output before administering an additional dose.
Before repeat administration, ensure that:
Respiratory rate is at least 16 per minute.
Patellar reflexes are present.
Urinary output is at least 30 mL per hour over 4 hours.
WITHHOLD OR DELAY DRUG IF:
Respiratory rate falls below 16 per minute.
Patellar reflexes are absent.
Urinary output falls below 30 mL per hour over preceding 4 hours.
–
If urine output is less than 30 mL per hour:
o
Withhold magnesium sulfate and infuse IV fluids (normal saline or Ringer’s
lactate) at 1 L in 8 hours;
o
Monitor for the development of pulmonary edema.
Keep antidote ready
In case of respiratory arrest:
-
Assist ventilation (mask and bag, anesthesia apparatus, intubation).
-
Give calcium gluconate 1 g (10 mL of 10% solution) IV slowly until respiration
begins to antagonize the effects of magnesium sulfate.
BOX 2 Maintenance dose for magnesium sulfate for management of severe preeclampsia and eclampsia
1. Give 5 g magnesium sulfate (50% solution) + 1 mL lignocaine 2% IM every 4 hours
into alternate buttocks.
34
o
Take one 20 mL sterile syringe.
o
Draw 5 ampoules of MgSO4 50% (10 mL = 5 gm) into the syringe.
o
Add 1 mL of 2% Lignocaine in the syringe.
o
Verify in which buttock the last magnesium sulfate injection was given.
o
Give deep IM injection in the alternate buttock.
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
Reference manual
Continue treatment with magnesium sulfate for 24 hours after
delivery or the last convulsion, whichever occurs last.
If magnesium sulfate is not available, diazepam may be used although there is a
greater risk for neonatal respiratory depression because diazepam passes the placenta
freely. A single dose of diazepam to abort a convulsion seldom causes neonatal respiratory
depression. Long-term continuous IV administration increases the risk of respiratory
depression in babies who may already be suffering from the effects of utero-placental
ischemia and preterm birth. The effect may last several days. Administration of diazepam is
outlined in Box 3.
BOX 3 Diazepam schedules for severe pre-eclampsia and eclampsia
Note: Use diazepam only if magnesium sulfate is not available.
Intravenous administration
Loading dose
Administer diazepam 10 mg IV slowly over 2 minutes.
If convulsions recur, repeat loading dose.
Maintenance dose
Diazepam 40 mg in 500 mL IV fluids (normal saline or Ringer’s lactate) titrated to
keep the woman sedated but rousable.
Maternal respiratory depression may occur when dose exceeds 30 mg in 1 hour:
-
Assist ventilation (mask and bag, anesthesia apparatus, intubation), if
necessary.
Do not give more than 100 mg in 24 hours.
Rectal administration (Give diazepam rectally when IV access is not possible.)
Loading dose
Load a 10 mL syringe with 20 mg of diazepam.
Remove the needle, lubricate the barrel and insert the syringe into the rectum to
half its length.
Discharge the contents and leave the syringe in place, holding the buttocks
together for 10 minutes to prevent expulsion of the drug.
If convulsions are not controlled within 10 minutes:
Administer an additional 10 mg per hour or more, depending on the size of the
woman and her clinical response.
Antihypertensive drugs
Recommendations: Antihypertensive therapy for severe hypertension (diastolic
pressure is 110 mm Hg or more)
If the diastolic blood pressure (dBP) is 110 mm Hg or more, give antihypertensive
drugs. The goal is to keep the diastolic pressure between 90 mm Hg and 100 mm Hg to
prevent cerebral hemorrhage. Labetolol and nifedipine are the drugs of choice.
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
35
Table xx. Description of anti-hypertensive drugs
Nifedipine
Labetalol
Hydralazine
Mode of
action
Calcium channel
blocker causing
dilatation of small
arteries
Combined alpha and betablocker. Less likely to reduce
utero-placental perfusion than
beta-blockers and may improve
cerebral circulation
Smooth muscle relaxant,
causing vasodilatation
Dose
10 mg orally (modified
release)
200 mg orally
OR
50 mg IV over 1 minute
10 mg IV slowly
Onset of
action
20 minutes
Oral: 30 minutes
IV: 5 minutes
5-20 minutes
4-6 hours
Oral: 8-12 hours (dose
dependent)
I.V.: 2-18 hours (dose
dependent; based on single and
multiple sequential doses of
0.25-0.5 mg/kg with
cumulative dosing up to 3.25
mg/kg)
1 to 4 hours
15 - 20 minutes if
inadequate
response to first
dose
If BP still uncontrolled:
Repeat 50 mg boluses to
maximum of 200 mg
OR
Start an IV infusion: 20
mg/hour, doubling dose at
half hourly intervals as
required to a maximum of
160 mg/hour
5–10 mg IV every 30
min
OR
0.5–10mg/hr IV
Maximum
dose
20 mg three times
per day
IV bolus: maximum of 200
mg
IV infusion: maximum of 160
mg/hour
The usual oral dose is 100
mg, twice a day, and may be
increased weekly to a total
of 800 mg, three times a day
(maximum of 2.4 g/day)
Maximum of 20mg IV
Maximum of 30 mg IM
Contraindications
Aortic stenosis
Risk of bronchospam in
asthmatics
Heart failure
Mitral valve rheumatic
heart disease
Duration of
action
Repeat
dose
Side
effects
36
Tachycardia
Cutaneous flushing
headache
Bradycardia (monitor pulse
and ensure it is greater than
or equal to 60 beats/minute)
Tachycardia
Chest tightness
Abdominal pain
Headache
Associated with more
maternal and perinatal
adverse effects than
other drugs
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
Reference manual
Initial antihypertensive therapy should be with labetalol, nifedipine capsules, nifedipine PA
tablets, or hydralazine.
-
Give nifedipine 5 mg under the tongue / 10 mg by mouth:
If response is inadequate (diastolic pressure remains above 110 mm Hg) after
15 - 20 minutes, give an additional 10 mg under the tongue.
OR
-
Give labetolol 50 mg IV over 1 minute / 200 mg by mouth
If response is inadequate (diastolic blood pressure remains above 110 mm Hg)
after 10 minutes:
o
Repeat 50 mg boluses to maximum of 200 mg
OR
o
Start an IV infusion: 20 mg/hour, doubling dose at half hourly intervals as
required to a maximum of 160 mg/hour
The usual oral dose is 100 mg, twice a day, and may be increased weekly to a
total of 800 mg, three times a day.
OR
-
Give hydralazine. Start with 5 mg IV; repeat 5–10 mg IV every 30 min, or 0.5–
10mg/hr IV, to a maximum of 20mg IV (or 30 mg IM)
MgSO4 is not recommended as an antihypertensive agent.
Continuous fetal heart rate (FHR) monitoring is advised until BP is stable.
Nifedipine and MgSO4 can be used contemporaneously.
Angiotensin-converting enzyme inhibitors (captopril) are contraindicated during
pregnancy
Diuretics (furosemide, hydrochlorothiazide) are contraindicated for use as an antihypertensive agent in pregnancy in settings in which uteroplacental perfusion is already
reduced (pre-eclampsia and intrauterine growth restriction).
Delivery
Delivery should take place as soon as the woman’s condition has stabilized. Delaying
delivery to increase fetal maturity will risk the lives of both the woman and the fetus.
Delivery should occur regardless of the gestational age.
In severe pre-eclampsia, delivery should occur within 24 hours of
the onset of symptoms. In eclampsia, delivery should occur
within 12 hours of the onset of convulsions.
Assess the cervix.
o
If the cervix is favorable (soft, thin, partly dilated), rupture the membranes
with an amniotic hook and induce labor using oxytocin or prostaglandins.
o
If the cervix is unfavorable (firm, thick, closed) and the fetus is alive, deliver
by cesarean operation.
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
37
Plan to delivery by cesarean operation if:
o
vaginal delivery is not anticipated within 12 hours (for eclampsia) or 24 hours
(for severe pre-eclampsia), deliver by cesarean operation
o
there are fetal heart rate abnormalities (less than 100 or more than 180
beats per minute), deliver by cesarean operation.
Do not use local anesthesia or ketamine in women with pre-eclampsia or
eclampsia.
Note: If cesarean operation is performed, ensure that:
-
Coagulopathy has been ruled out;
-
Safe general anesthesia is available. Spinal anesthesia is associated with the
risk of hypotension.
If safe anesthesia is not available for cesarean operation or if the fetus is dead
or too premature for survival:
-
Aim for vaginal delivery;
-
If the cervix is unfavorable (firm, thick, closed), ripen the cervix using
misoprostol, prostaglandins or a Foley catheter.
Postpartum care
Anticonvulsive therapy should be maintained for 24 hours after delivery or the last
convulsion, whichever occurs later.
Continue antihypertensive therapy as long as the diastolic pressure is 110 mm Hg or
more.
Continue to monitor urine output.
Ensure counseling about family planning in the postpartum period.
Referral for tertiary level care
Consider referral of women who have:
oliguria (urine output is less than 15 ml/hour) that persists for 48 hours after delivery;
coagulation failure [e.g. coagulopathy or hemolysis, elevated liver enzymes and low
platelets (HELLP) syndrome];
persistent coma lasting more than 24 hours after convulsion.
38
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
Reference manual
Management during a convulsion / fit
Stages of an eclamptic fit
Convulsions with signs of pre-eclampsia indicate eclampsia. Occasionally convulsions occur
when there is no hypertension, only proteinuria. Other women may have raised blood
pressure and proteinuria, but only one or two of the signs of severe pre-eclampsia when a
fit occurs.
Convulsions associated with eclampsia:
Can occur regardless of the severity of hypertension
Are difficult to predict and typically occur in the absence of headache or visual changes
May recur in rapid sequence, as in status epilepticus, and may end in death
Will not be observed if the woman is alone
The following are stages of an eclamptic fit (WHO, 2008):
1. Premonitary stage: Lasts 10–20 seconds, during which:
o
the eyes roll or stare
o
the face and hand muscles may twitch
2. Tonic stage: Lasts up to 30 seconds, during which:
o
the muscles go into violent spasm
o
the fists are clenched and arms and legs are rigid
o
the diaphragm (which is a muscle separating the chest from the abdomen) is
in spasm, so that breathing stops and the color of the skin becomes blue or
dusky (cyanosis)
o
the back may be arched
o
the teeth are clenched
o
the eyes bulge
3. Clonic stage: Lasts 1–2 minutes and is marked by:
o
violent contraction and relaxation of the muscles
o
increased saliva causes “foaming” at the mouth and there is a risk of
inhalation
4. Coma stage: May last for minutes or hours. The woman is unconscious and often
breathes noisily. The cyanosis fades but her face may still be swollen and congested.
Further fits may occur.
The woman may die after only one or two fits.
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
39
Management during a convulsion
Immediate management during a convulsion
1. SHOUT FOR HELP to urgently mobilize available personnel.
2. Gather equipment (airway, suction, mask and bag, oxygen)
3. Airway: Turn the woman onto her left side to reduce the risk of aspiration of
secretions, vomit and blood.
4. Ensure the woman’s airway is open
5. Breathing: Assess breathing
6. If the woman is not breathing, begin resuscitation measures
7. Give oxygen at 4–6 L per minute by mask or cannulae.
8. Circulation: Evaluate pulse
9. If absent, initiate CPR and call arrest team
10. Protect her from injury but do not actively restrain.
Care after the convulsion
1. Aspirate the mouth and throat as necessary.
2. Encourage the woman to lie on her side to reduce the risk of aspiration of
secretions, vomit and blood.
3. Ensure the woman’s airway is open.
4. Observe color for cyanosis and need for oxygen
5. If available, continue oxygen at 4–6 L per minute by mask or cannulae.
6. Check for aspiration: Lungs should always be auscultated after the convulsion has
ended
7. Check vital signs and fetal heart rate
8. Start an intravenous of normal saline or Ringer’s lactate, if not yet started
9. Give anticonvulsive drugs (see Learning Guide for administering magnesium
sulfate), if not yet started or due
10. If diastolic blood pressure remains above 110 mm Hg, give antihypertensive
drugs
11. Insert an indwelling urinary catheter to monitor urine output and proteinuria, if
one has not yet been placed
12. Do a bedside clotting test, if not yet done
13. Never leave the woman alone. A convulsion followed by aspiration of vomit may
cause death of the woman and fetus.
14. Check for signs of labor (see Learning Guide for vaginal examination of a
pregnant woman)
15. If this was the woman’s first convulsion and eclampsia has not yet been
diagnosed, make a differential diagnosis.
16. Provide specific management based on diagnosis. A small proportion of women
with eclampsia have normal blood pressure. Treat all pregnant/postpartum
women with convulsions as if they have eclampsia until another diagnosis is
confirmed
17. Record drug administration and findings on the woman’s record.
40
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
Reference manual
Share findings with the woman
1. Share your findings with the woman and, as appropriate, her partner or family
member
2. Discuss any complications / problems detected:
Complications / problems
Possible cause(s)
3. Explain management, based on diagnosis, and the importance for pregnancy,
labor, and delivery
4. If the woman has to be referred, explain the need for referral
5. Check the woman’s understanding of findings and next steps and answer any
questions.
6. Inform the family that the woman should never be left alone.
Differential diagnosis of convulsions
Carefully examine the woman and order tests / examinations to confirm a diagnosis. If a
pregnant woman presents with convulsions/fits, it is best to begin treatment for eclampsia
while waiting to rule out other causes.
A small proportion of women with eclampsia have normal
blood pressure. Treat all pregnant/postpartum women with
convulsions as if they have eclampsia until another diagnosis
is confirmed.
There are many causes for convulsions/fits in pregnancy. It is important to understand them
well, because their management differs. Table XX provides an overview of diagnostic criteria
for the different disorders in pregnancy that may lead to convulsions/fits.
Table XX. Differential diagnosis of convulsions/fits in pregnancy
Presenting symptom and other
symptoms and signs typically
present
Convulsions / Fits
Diastolic BP 90 mm Hg or more
after 20 weeks gestation
Proteinuria 2+ or more
Trismus (difficulty opening
mouth and chewing)
Symptoms and signs sometimes
present
Coma (unconscious)
Other signs and symptoms of severe
pre-eclampsia
- Headache (increasing frequency,
unrelieved by regular analgesics)
- Blurred vision
- Oliguria (passing less than 400 mL
in 24 hours)
- Upper abdominal pain (epigastric
pain or pain in upper right
quadrant)
- Pulmonary edema
Spasms of face, neck, trunk
Arched back
Spontaneous violent spasms
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
Probable
diagnosis
Eclampsia
Tetanus
41
Presenting symptom and other
symptoms and signs typically
present
Convulsions/fits
Past history of convulsions/fits
Normal blood pressure
Fever
Chills/rigor
Headache
Muscle/joint pain
Coma
Anemia
Headache
Stiff neck
Photophobia
Fever
Symptoms and signs sometimes
present
Probable
diagnosis
Epilepsy
Convulsions/Fits
Jaundice
Severe /
complicated
malaria
Meningitis or
Encephalitis
Convulsions/Fits
Confusion
Drowsiness
Coma
Table xx gives of an overview of tests to consider performing to rule out or confirm a
diagnosis in a pregnant woman presenting with hypertension during pregnancy or in the
postpartum period
42
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
Reference manual
Table xx. Tests to make a differential diagnosis of convulsions / fits during pregnancy and the postpartum.
Blood pressure
Urine for protein
Eclampsia
Usually raised in cases
of eclampsia
Will contain protein
Epilepsy
Platelet count
Bedside
clotting test
Renal function
tests
EEG (electroencephalogram)
Meningitis
Urine may show
temporary proteinuria
after a fit, but
otherwise testing
shows no abnormality.
In cerebral malaria,
more than 5 per cent
of circulating red cells
will be parasitized
Blood film to
exclude malaria
Liver enzymes
and function tests
Cerebral malaria
Will be elevated in
Eclampsia indicating
liver damage
Often low in preeclampsia/eclampsia
Coagulation defects may
be present in eclampsia
Urea may be elevated in
eclampsia indicating
kidney damage.
Creatinine clearance and
serum proteins may be
decreased
Blood urea is normal.
May show typical
abnormalities
Examination of
cerebrospinal
fluid
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 December 2010
The cerebro-spinal fluid (CSF)
is under increased pressure
The fluid looks cloudy in coccal
forms of meningitis but is clear
in viral meningitis
The causal organism is usually
found under bacteriological
examination and the cell count
is increased
Protein is increased, sugar and
chlorides decreased.
43
44
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
Reference manual
Birth preparedness and complication readiness
When delays occur in recognizing problems and referring women to appropriate health care
facilities, the result can lead to maternal and newborn deaths. One solution to combat these
problems is to work with the pregnant woman and her family to develop two plans: a birthpreparedness plan and a complication-readiness plan (Jhpiego/MNH, 2001).
Birth-preparedness plan
Having a birth plan can reduce delayed decision-making and increase the probability of
timely care. A birth-preparedness plan is an action plan made by the woman, her family
members, and the health care provider. Often this plan is not a written document, but
instead is an ongoing discussion between all concerned parties to ensure that the woman
receives the best care in a timely manner. Each family should have the opportunity to make
a plan for the birth. Health care providers can help the woman and her family to develop
birth-preparedness plans and discuss birth-related issues. Work with the woman to:
Make plans for the birth:
Discuss the idea of a birth plan and what to include during the first visit.
If planning a home delivery with a skilled birth attendant, discuss access to a safe
delivery kit consisting of 1) a piece of soap for cleaning the birth attendant’s hands
and the woman’s perineum, 2) a plastic sheet about one square meter for use as a
clean delivery surface, 3) clean string for tying the umbilical cord (usually two
pieces), and 4) a clean razor blade for cutting the cord.
Inquire about the birth-preparedness plan during the third or fourth antenatal visits.
Ask if arrangements are made for a skilled birth attendant and the birth setting
during the antenatal visit in the eighth month.
Note: In some cultures, superstition surrounds buying items for an
unborn baby. If this is not the case, families can prepare for the birth
by buying baby supplies such as blankets, diapers, and clothes.
Make birth-related decisions:
Where to give birth.
Who will be the skilled birth attendant.
How to contact the provider.
How to get to the place of birth.
Who will be the birth companion.
Who will take care of the family while the woman is absent.
How much money is needed and how to access these funds.
Prepare for the birth:
Discuss items needed for the birth (perineal pads/cloths, soap, clean bed sheets,
etc.) on the third antenatal visit.
Confirm necessary items are gathered near the due date.
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
45
Save money:
Discuss why and how to save money in preparation for the birth during the first visit.
Discuss how to plan to make sure that any funds needed are available at birth.
Check that the woman and her family have begun saving money or that they have
ways to access necessary funds.
Note: Encourage the family to save money so necessary funds are
available for routine care during pregnancy and birth. Assess
financial needs with the women as well as sources for accessing
these funds so they are available before labor.
Complication-readiness plan
The complication-readiness plan is an action plan that outlines steps that can be discussed
and determined prior to an emergency. Developing this plan helps the family to be prepared
for and respond quickly when the woman or newborn has a complication and needs medical
care. It is important that a complication-readiness plan is prepared with the woman and her
chosen family members. Unless others are involved, the woman may have difficulties
putting the plan into action should complications occur for her or her baby.
Recognize danger signs
Women, family members, and community caregivers must know the signs of lifethreatening complications. Many hours can be lost from the time a complication is
recognized until the time arrangements are made for the woman to reach help. For
eclampsia, the woman and her baby may die after only one or two eclamptic fits. It is
critical to reduce the time needed to recognize problems and make arrangements to receive
care at the most appropriate level of care. Women, family members, and community
caregivers must know the signs of life-threatening complications.
Maternal danger signs include:
Vaginal bleeding (any vaginal bleeding during pregnancy; heavy vaginal bleeding or a
sudden increase in vaginal bleeding during the postpartum period).
Breathing difficulties.
46
Fever.
Swelling of hands and face.
Severe abdominal pain (particularly epigastric pain).
Severe headache/blurred vision.
Convulsions or loss of consciousness.
Foul-smelling discharge from vagina, tears, and incisions.
Calf pain with or without swelling.
Night blindness
Verbalization or behavior indicating she may hurt the baby or herself.
Hallucinations.
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
Reference manual
Newborn danger signs include:
Breathing problems
Feeding difficulties or not sucking.
Feels cold or has fever.
Redness, swelling, or pus from eyes or around the cord or umbilicus.
Convulsions or fits.
Jaundice (yellow skin).
Save money
Similar to the birth preparedness plan, the family should be encouraged to save money so
necessary funds are available for emergencies. In many situations, women either do not
seek or receive care because they lack funding to pay for services.
Choose a decision-maker in case of emergency
In many families, one person is the primary decision-maker. Too often, other members of
the family do not feel they can make decisions if that person is absent. This can result in
death when an emergency occurs and the primary decision-maker is absent. It is important
to discuss how the family can make emergency decisions without disrupting or offending
cultural and family values. If possible, find out which family member can make a decision in
the absence of the chief decision-maker.
Have an emergency transportation plan
Too many women and newborns die because they suffer serious complications and do not
have access to transportation to the type of health care facility that can provide needed
care. Each family should develop a transportation plan during the woman’s early pregnancy
in case the woman experiences complications and urgently needs a higher level of care. This
plan should be prepared during pregnancy and after giving birth, either before discharge
from the health facility or immediately after returning home. The plan should address the
following:
Where to go if complications arise.
How to get to the next level of care in case of an emergency.
Who in the family will accompany the woman.
Have an emergency blood donation plan
Many health care facilities lack an inadequate, safe blood supply for transfusions. After
birth, women are more likely to need blood transfusions because the complications they
experience from birth lead to blood loss. For these reasons, it is extremely important
that the woman and her family determine, before labor has started, blood donors that
can be available if needed.
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
47
48
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
Reference manual
References
1. Cnossen JS, Vollebregt KC, de Vrieze N, ter Riet G, Mol BWJ, Franx A, Khan KS, and van der Post
JAM. Accuracy of mean arterial pressure and blood pressure measurements in precicting preeclampsia: systematic review and meta-analysis. BMJ 2008;336;1117-1120.
2. Conde-Agudelo A, Belizan JM. Risk factors for pre-eclampsia in a large cohort of Latin American
and Caribbean women. Br J Obstet Gynaecol 2000; 107: 75–83.
3. Coomarasamy A, Braunholtz D, Song F, et al. Individualizing use of aspirin to prevent preeclampsia: a framework for clinical decision making. BJOG 2003; 110:882–888.
4. Côté A-M, Firoz T, Liston RM, Magee LA, Brown MA, Lam E, von Dadelszen P. Diagnostic accuracy
of urinary spot protein:creatinine ratio for proteinuria in hypertensive women: systemic review.
BMJ 2008;336;1003-1006.
5. Craici I, Wagner S, Garovic VD. Pre-eclampsia and future cardiovascular risk: formal risk factor or
failed stress test?. Ther Adv Cardiovasc Dis. Aug 2008;2(4):249-59. [Medline].
6. Dekker G, Sibai B. Primary, secondary, and tertiary prevention of pre-eclampsia. Lancet 2001;
357: 209–15.
7. Duley L Pre-eclampsia and the hypertensive disorders of pregnancy. Med Bull (2003) 67 (1): 161176.
8. Duley L, Gulmezoglu AM, Henderson-Smart DJ. Magnesium sulphate and other anticonvulsants for
women with pre-eclampsia. In: The Cochrane Library, Issue 1, 2003. Oxford: Update Software
9. Duley L, Gulmezoglu AM. Magnesium sulphate versus lytic cocktail for eclampsia. In: The
Cochrane Library, Issue 1, 2003. Oxford: Update Software
10. Duley L, Henderson-Smart D. Magnesium sulphate versus diazepam for eclampsia. In: The
Cochrane Library, Issue 1, 2003. Oxford: Update Software
11. Duley L, Henderson-Smart D. Magnesium sulphate versus phenytoin for eclampsia. In: The
Cochrane Library, Issue 1, 2003. Oxford: Update Software
12. Duley L, Henderson-Smart D. Reduced salt intake compared to normal dietary salt, or high intake,
in pregnancy. In: The Cochrane Library, Issue 1, 2003. Oxford: Update Software
13. Duley L, Henderson-Smart D, Meher S. Altered dietary salt for preventing pre-eclampsia, and its
complications. In: The Cochrane Library, Issue 2, 2007. Chichester, UK: John Wiley & Sons Ltd.
Search date 2005; primary sources Cochrane Pregnancy and Childbirth Group Trials Register, The
Cochrane Library, and Embase.
14. Gabbe. Hypertension. In: Obstetrics: Normal and Problem Pregnancies. 5th ed. Churchill
Livingstone, An Imprint of Elsevier; 2007:[Full Text].
15. Gauer R, Atlas M. Does low-dose aspirin reduce preeclampsia and other maternal-fetal
complications? Journal of Family Physicians. vol 57, 1 / January 2008.
16. Gibbs RS, Karlan BY, Haney AF, Nygaard I. Danforth’s Obstetrics and Gynecology, Tenth Edition.
Lippincott, Williams & Wilkins: Philadelphia, 2008.
17. Higgins JR, de Swiet M. Blood-pressure measurement and classification in pregnancy. Lancet
2001; 357: 131–35.
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
49
18. Hofmeyr GJ, Atallah ÁN, Duley L. Calcium supplementation during pregnancy for preventing
hypertensive disorders and related problems (Review). 2009 The Cochrane Collaboration.
19. Hofmeyr G J, Belfort M. Proteinuria as a predictor of complications of pre-eclampsia BMC Medicine
2009, 7:11 doi:10.1186/1741-7015-7-11. This article is available from:
http://www.biomedcentral.com/1741-7015/7/11
20. Jhpiego and Maternal and Neonatal Health (MNH). Birth Preparedness and Complication
Readiness. Baltimore, MD: JHPIEGO/MNH; 2001.
21. Koopmans CM, Bijlenga D, Groen H, Vijgen SMC, Aarnoudse JG, Bekedam DJ, van den Berg PP, de
Boer K, Burggraaff JM, Bloemenkamp KWM, Drogtrop AP, Franx A, de Groot CJM, Huisjes AJM,
Kwee A, van Loon AJ, Lub A, Papatsonis DNM, van der Post JAM, Roumen FJME, Scheepers HCJ,
Willekes C, Mol BWJ, van Pampus MG, for the HYPITAT study group. Induction of labour versus
expectant monitoring for gestational hypertension or mild pre-eclampsia after 36 weeks’ gestation
(HYPITAT): a multicentre, open-label randomised controlled trial. www.thelancet.com Published
online August 4, 2009 DOI:10.1016/S0140-6736(09)60736-4.
22. Kramer MS. Aerobic exercise for women during pregnancy. In: The Cochrane Library, Issue 1,
2003. Oxford: Update Software
23. Kramer MS. Balanced protein/energy supplementation in pregnancy. In: The Cochrane Library,
Issue 1, 2003. Oxford: Update Software.
24. Kramer MS. Energy/protein restriction for high weight-for-height or weight gain during pregnancy.
In: The Cochrane Library, Issue 1, 2003. Oxford: Update Software
25. Kramer MS. Isocaloric balanced protein supplementation in pregnancy. In: The Cochrane Library,
Issue 1, 2003. Oxford: Update Software.
26. Kramer MS. Regular aerobic exercise during pregnancy. In: The Cochrane Library, Issue 1, 2003.
Oxford: Update Software
27. Magee LA, Ornstein MP, von Dadelszen P. Management of hypertension in pregnancy. BMJ 1999;
318: 1332–36.
28. Magpie Trial Collaborative Group: Do women with pre-eclampsia, and their babies, benefit from
magnesium sulphate? The Magpie Trial: A randomised placebo-controlled trial. Lancet 2002,
359(9321):1877-90.
29. The Magpie Trial Follow-up Study Collaborative Group: The Magpie Trial: a randomised trial
comparing magnesium sulphate with placebo for pre-eclampsia. Outcome for children at 18
months. BJOG 2007, 114(3):289-299.
30. The Magpie Trial Follow-up Study Collaborative Group: The Magpie Trial: a randomised trial
comparing magnesium sulphate with placebo for pre-eclampsia. Outcome for women at two years.
BJOG 2007, 114(3):300-309
31. Mahomed K. Zinc supplementation in pregnancy. In: The Cochrane Library, Issue 1, 2003. Oxford:
Update Software
32. Makrides M, Crowther CA. Magnesium supplementation in pregnancy. In: The Cochrane Library,
Issue 1, 2003. Oxford: Update Software
33. Makrides M, Duley L, Olsen SF. Fish oil, and other prostaglandin precursor, supplementation
during pregnancy to improve outcomes in pre-eclampsia, preterm birth, low birth weight and small
for gestational age. In: The Cochrane Library, Issue 1, 2003. Oxford: Update Software
34. Marshall M, Buffington S. Life-saving skills manual for midwives. 3rd edition. Washington, DC:
American College of Nurse Midwives (ACNM), 1998
50
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
Reference manual
35. Marti JJ, Hermann U. Immunogestosis: a new etiologic concept of “essential” EPH gestosis, with
special consideration of the primigravida patient. Am J Obstet Gynecol 1977; 128: 489–93.
36. Munjuluri N, Lipman M, Valentine A, Hardiman P, Maclean AB (November 2005). "Postpartum
eclampsia of late onset". BMJ 331 (7524): 1070–1.
37. National High Blood Pressure Education Program Working Group on High Blood Pressure in
Pregnancy. Am J Obstet Gynecol. 2001 Aug;185(2):522-3.
38. POPPHI. Planning tool for expanding access to active management of the third stage of labor
(AMTSL): A guide for program managers and donors. Seattle: PATH; 2008.
39. Rasmussen KM and Yaktine AL, Editors (Committee to Reexamine IOM Pregnancy Weight
Guidelines; Institute of Medicine; National Research Council). Weight Gain During Pregnancy:
Reexamining the Guidelines. The National Academies Press: Washington, D.C., 2009.
40. Rivers EP. Pre-eclampsia, eclampsia, and other hypertensive disorders of pregnancy. In: The
Clinical Practice of Emergency Medicine. 2nd ed. 1996:315-21.
41. Roberts J M, Cooper D W. Pathogenesis and genetics of pre-eclampsia. Lancet 2001; 357: 53–56.
42. Robillard PY, Dekker GA, Hulsey TC. Revisiting the epidemiological standard of pre-eclampsia:
primigravidity or primipaternity. Eur J Obstet Gynecol Reprod Biol 1999; 84: 37–41.
43. Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet 2005; 365: 785–99.
44. Smyth R, Spark P, Armstrong N and Duley L. Magpie Trial in the UK: methods and additional data
for women and children at 2 years following pregnancy complicated by pre-eclampsia. BMC
Pregnancy and Childbirth 2009, 9:15 doi:10.1186/1471-2393-9-15. This article is available from:
http://www.biomedcentral.com/1471-2393/9/15
45. Society of Obstetricians and Gynecologists of Canada. Diagnosis, Evaluation, and Management of
the Hypertensive Disorders of Pregnancy. JOGC March 2008: Volume 30, Number 3 supplement 1.
46. Thangaratinam S, SCoomarasamy A, O'Mahony F, SSharp S, Zamora J, Khan KS and S Ismail
KMK, Estimation of proteinuria as a predictor of complications of pre-eclampsia: a systematic
review. BMC Medicine 2009, 7:10 doi:10.1186/1741-7015-7-10. This article is available from:
http://www.biomedcentral.com/1741-7015/7/10.
47. Villar J, Abalos E, Nardin JM, et al. Strategies to prevent and treat pre-eclampsia: evidence from
randomized controlled trials. Semin Nephrol 2004; 24:607–615.
48. Vogel S, Rajaii R, Ottaviano G, Kim L, Amanda Yeaton-Massey, Aaron Caughey et al. Low-dose
aspirin for prevention of preeclampsia and its complications: a cost-effectiveness analysis.
American Journal of Obstetrics & Gynecology Vol. 201, Issue 6, Supplement, Page S218,
December 2009
49. Waugh JJS, Clark TJ, Divakaran TG, Khan KS, Kilby MD. Accuracy of Urinalysis Dipstick Techniques
in Predicting Significant Proteinuria in Pregnancy. ACOG: VOL. 103, NO. 4, APRIL 2004.
50. WHO. Managing complications in pregnancy and childbirth. WHO: Geneva, 2003.
51. World Health Organization (WHO) Department of Making Pregnancy Safer. Midwifery Education
Modules (2nd Edition): Managing eclampsia. Geneva, Switzerland: WHO; 2008.
52. World Health Organization (WHO) Mother-Baby Package: Implementing Safe Motherhood in
Countries. WHO/FHE/MSM/94.11. Geneva: WHO; 1994.
53. WHO and UNICEF. The world health report 2005 – Countdown to 2015 Decade Report (20002010_ with country profiles: Taking stock of maternal, newborn, and child survival. Geneva: WHO,
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011
51
2010. Available at: http://whqlibdoc.who.int/publications/2010/9789241599573_eng.pdf,
accessed 7 July 2010.
52
Prevention and management of pre-eclampsia and eclampsia
Version 7.0 / 10 January 2011