28_SWP_Maxi prep_KT

OHS026
Safe Work Procedure
Faculty/Division
Medicine
Document number
SOMS.CGM.SWP028
Initial Issue date
30/06/09 1.0
School/ Divisional Unit
School of Medical Sciences
Current version Current Version
Issue date
30/06/09
Next review date
30/06/12
The Writing Safe Work Procedures Guideline (OHS027) should be consulted to assist in the completion of this form.
Safe Work Procedure Title and basic description
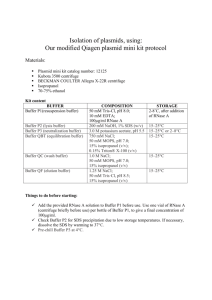
Title: Isolation of plasmid DNA
Description: Isolation of plasmid DNA from bacterial cell cultures using Qiafilter Maxiprep kit (Qiagen)
Associated risk assessment title and location: 28_RA_Maxi prep_KT
Describe the activity or process
Procedure adapted from the Qiafilter Plasmid Purification Handbook. Consult this handbook before using Maxiprep kit.
1. Prepare an appropriate volume of LB broth – 250 mL of LB should yield approximately 250 µg of a low-copy number plasmid.
All buffer volumes given are for a 250 mL culture. Consult kit handbook for volumes required for smaller cultures.
For 1 L of LB dissolve 10 g of tryptone and NaCl and 5 g of yeast extract in 1 L MilliQ water and autoclave.
2. Inoculate the prepared LB broth with the appropriate antibiotic – 250 µL of 10 mg/mL in 250 mL of culture gives an antibiotic concentration of 10 µg/mL.
3. Either directly inoculate the media by picking a single colony from an agar plate with a sterile toothpick or pipette tip and placing this directly into the media. This may be done over a flame to minimise contamination. Grow the cells for up to 24 hrs at 37° with vigorous shaking.
4. Alternatively, inoculate a 2-5 mL starter culture as described above and grow this for 8 hr at 37° with shaking. A 250 mL culture should be inoculated with 250 – 500 µL of starter culture and grown overnight.
5. Harvest the cells by centrifuging at 4000 g for 20 minutes at 4°.
6. Resuspend the pelleted cells in 10 mL Buffer P1.
7. Add 10 mL Buffer P2 and mix thoroughly until a homogeneous blue suspension is formed.
8. Incubate at room temperature for 5 mins.
9. During this time prepare the QIAfilter cartridge by screwing the cap into the outlet nozzle and placing the cartridge in a convenient tube.
10. Add 10 mL chilled Buffer P3 to the lysate and mix immediately until all blue colour has disappeared.
11. Immediately pour the lysate into the barrel of the QIAfilter cartridge and incubate at room temperature for 10 mins. Do not insert the plunger.
12. Prepare a QIAGEN-tip 500 by applying 10 mL of Buffer QBT to the column and allowing to empty by gravity flow.
13. Remove the cap from the QIAfilter cartridge and insert the plunger. Gently filter the lysate into the equilibrated column and allow the lysate to enter the resin by gravity flow.
14.
Wash the column with 2 × 30 mL of buffer QC.
15. Elute the DNA into a 50 mL glass centrifuge tube with 15 mL Buffer QF.
16. Precipitate DNA by adding 10.5 mL room temperature isopropanol to the eluate. Mix and immediately centrifuge at 12000 g for
40 mins at 4°. Decant the supernatant. Take care not to discard the pellet as it will be loosely attached to the wall of the tube.
17. Wash the pellet with 5 mL room temperature 70% ethanol and centrifuge at 12000 g for 15 min at 4°. Completely remove the supernatant and allow the pellet to air-dry for 5
– 10 mins.
18. Redissolve the DNA in nuclease-free water or TE buffer.
List all resources required including plant, chemicals, personal protective clothing and equipment, etc
QIAfilter Maxi Prep kit; Bioline orbital shaker incubator; Sorvall RC 5B Plus centrifuge; autoclave; pipettes; autoclaved pipette tips; lidded centrifuge bottles; centrifuge tubes; safety glasses; nitrile gloves; lab coat; heat resistant gloves; antibiotics; tryptone; NaCl; yeast extract; isopropanol; ethanol; nuclease-free water; TE buffer.
___________________________________________________________________________________________________________
___________
Page 1 of 2
Safe Work Procedure
Uncontrolled document when printed
Date Effective: 01/01/2007
Current Version: 1.2, 15/08/2007
List potential hazards and risk controls including specific precautions required
Autoclave – risk of burns; injury from incorrect operation of autoclave. Only individuals who have been trained in the correct use of the autoclave should perform this operation.
Do not remove items from autoclave until they’ve cooled to a safe temperature.
Use appropriate protective equipment when handling hot items, including heat-resistant gloves.
Burns from Bunsen burner
– only leave the Bunsen burning while in use. When no longer required turn off the gas.
Turn the Bunsen onto the more visible safety flame when not directly using it.
Centrifuge – risk of mechanical injury. Ensure rotor is correctly fitted in before use. Ensure centrifuge is balanced and the lid is firmly screwed in place. Only trained users should operate the centrifuge.
Orbital shaker incubator
– risk of mechanical injury. Ensure shaking has stopped before opening the incubator.
Bacteria
– risk of contamination from cultured bacteria. Always wear PPE when handling bacteria. Open agar plates or culture vessels where bacteria have been grown for the minimum time required. Dispose of contaminated waste correctly.
Ethanol
– flammable, risk of burns. Do not dispense ethanol near an open flame or source of ignition.
Isopropanol – flammable, risk of burns. Do not dispense isopropanol near an open flame or source of ignition.
List emergency shutdown instructions
In the event of an electrical emergency turn the centrifuge or incubator off at the unit/power point or, if it is unsafe to do so, hit the “Laboratory Power Emergency Shutdown” button.
In case of a fire, immediately turn gas off at the tap. Fire extinguishers are located in the laboratory.
Please notify the Laboratory Manager (Renee Szokolai x58497) of any incidents.
List clean up and waste disposal requirements
LB agar plates contaminated with bacteria should be sealed and disposed of in biological waste bins ready to be autoclaved before disposal.
Liquid media contaminated with bacteria should be disinfected with Betadine antiseptic solution or (4%) bleach solution and stored in a 20 L screw top waste container for collection.
List legislation, standards and codes of practice used in the development of the SWP
NSW OHS Act 2000
NSW OHS Regulation 2001
Australian Standard AS2243.3-2002. Safety in laboratories. Part 3: Microbiological aspects and containment facilities.
Australian Standard AS2243.6-1990. Safety in laboratories. Part 6: Mechanical Aspects.
Australian Standard AS2243.7-1991. Safety in laboratories. Part 7: Electrical Aspects.
Safe Work Procedure Form (OHS026)
Supervisory approval, training, and review
Supervisor: Peter Gunning Signature:
Plant custodian: Signature
List competency required – qualifications, certificates, licencing, training - eg course or instruction:
Training as per Training Needs Analysis, Induction to Lab, Training in this SWP
___________________________________________________________________________________________________________
___________
Page 2 of 2
Safe Work Procedure
Uncontrolled document when printed
Date Effective: 01/01/2007
Current Version: 1.2, 15/08/2007