Teacher Background: Kool-Aid Chromatography
advertisement

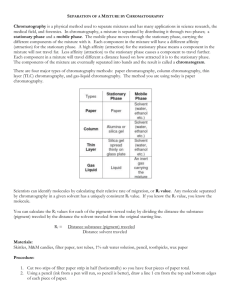
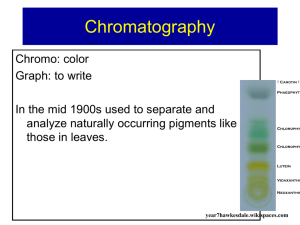
Teacher Background: Kool-Aid Chromatography Students should conclude that the drink mix is a mixture. The different colors represent different components of the mixture that separate based on differences in their physical properties. Early Egyptians separated dye pigments by spotting the dyes onto papyrus leaves. The tips of the papyrus leaves were dipped into water. As the water carried the sample with it along the surface of the leaf, the various color pigments in the dye separated and were deposited on the leaf’s surface. Pictures as well as additional information about this unusual plant can be found at Papyrus. The name “chromatography” was derived from the Greek words “chroma,” meaning “color,” and “graphein,” meaning “to write.” The name has stuck even though colors are incidental and have no bearing on the principles of the method. The graphic below is an example of a chromatogram produced using paper chromatography to separate the dyes in a green felt tip marker. All chromatographic separations are based on the dispersal of sample components between two phases. The stationary phase is either a solid or liquid which remains fixed in the system. The mobile phase is either a liquid or gas that moves through the system in a definite direction. For the early Egyptians, the papyrus leaves served as a stationary phase and water was used as the mobile phase. When the dyes in a felt marker or ink pen are separated the water is the mobile phase and the paper is the stationary phase. The separations in chromatography depend both on the properties of the molecules to be separated and the properties of the solid and liquid phase. “Like dissolves like” applies here. The components of the mixture to be separated will spend the greatest time in the phase with which they have the most similar properties. If this is the stationary phase the material will not move quickly. Care must be taken to choose solid and liquid phases that will separate but not react with any of the components in the mixture. Chromatography allows scientists to separate, isolate, identify, and quantify the components of a mixture. Mastering Matter 1 http://MathInScience.Info