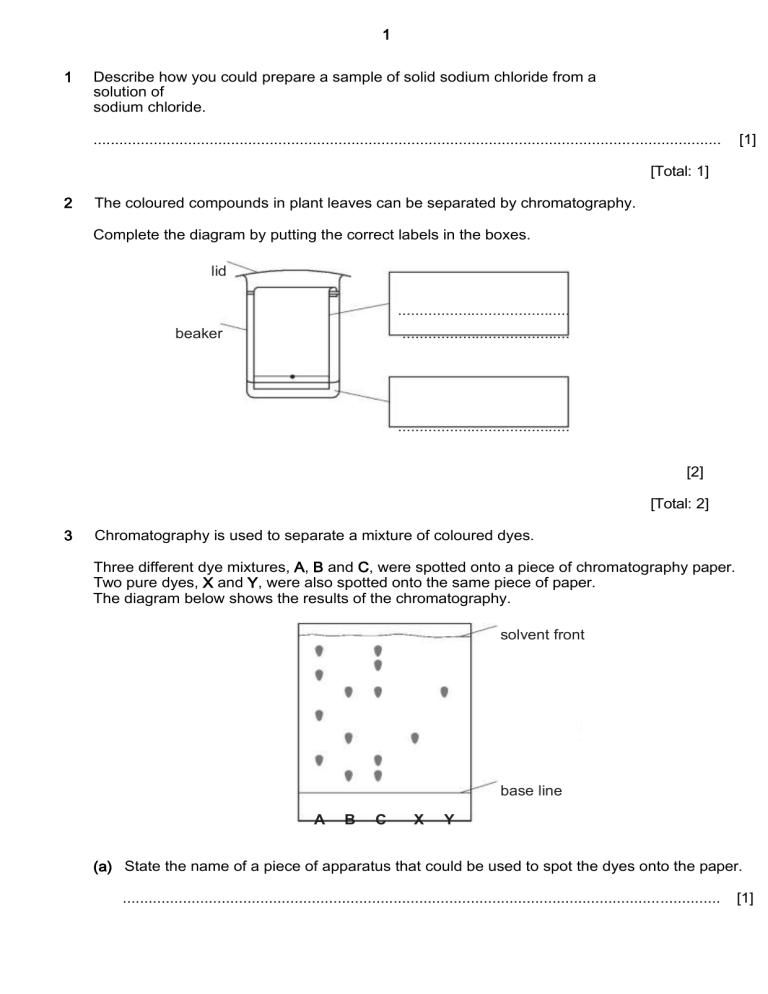
1 1 Describe how you could prepare a sample of solid sodium chloride from a solution of sodium chloride. .................................................................................................................................................. [1] [Total: 1] 2 The coloured compounds in plant leaves can be separated by chromatography. Complete the diagram by putting the correct labels in the boxes. lid ........................................ beaker ....................................... ........................................ [2] [Total: 2] 3 Chromatography is used to separate a mixture of coloured dyes. Three different dye mixtures, A, B and C, were spotted onto a piece of chromatography paper. Two pure dyes, X and Y, were also spotted onto the same piece of paper. The diagram below shows the results of the chromatography. solvent front base line A B C X Y (a) State the name of a piece of apparatus that could be used to spot the dyes onto the paper. ........................................................................................................................................... [1] 2 (b) Suggest why the base line was drawn in pencil and not in ink. ........................................................................................................................................... [1] (c) Which dye mixture contains both dye X and dye Y? ........................................................................................................................................... [1] (d) Which dye mixture does not contain dye X or dye Y? ........................................................................................................................................... [1] (e) In which mixture, A, B or C, has the greatest number of dyes been separated? ........................................................................................................................................... [1] [Total: 5] 4 A list of techniques used to separate mixtures is given below. chromatography evaporation filtration crystallisation diffusion fractional distillation dissolving simple distillation (a) From the list, choose the most suitable technique to separate the following. water from sea-water ...................................................................................................... sand from a mixture of sand and water ................................................................ ethanol from a mixture of ethanol and propanol ............................................................. iron filings from a mixture of iron filings and water .......................................................... (b) Describe how you would obtain a pure sample of copper(II) sulfate-5-water crystals from a mixture of copper(II) sulfate-5-water with copper(II) oxide using some of the techniques listed above. [5] ........................................................................................................................................... ........................................................................................................................................... ........................................................................................................................................... ........................................................................................................................................... ........................................................................................................................................... [Total: 9] [4] 3 5 Air is a mixture. Nitrogen and oxygen are the two most common gases in air. (a) What is meant by the term mixture? ........................................................................................................................................... ........................................................................................................................................... [1] (b) State the percentage of oxygen, to the nearest whole number, in clean dry air. ........................................................................................................................................... [1] (c) Describe the steps in the industrial process which enables nitrogen and oxygen to be separated from clean dry air. Use scientific terms in your answer. ........................................................................................................................................... ........................................................................................................................................... ...........................................................................................................................................[3] (d) Which physical property of nitrogen and oxygen allows them to be separated? ........................................................................................................................................... [1] [Total: 6] Crystallization exploits difference in which factors? a) Specific heat b) Boiling point c) Melting point d) Bubble point 4 6 Describe how simple distillation is used to separate water from an aqueous solution of sodium sulfate. In your answer, refer to: • the apparatus used, • changes in state, • differences in boiling points. You may use a diagram. .................................................................................................................................................. .................................................................................................................................................. .................................................................................................................................................. .................................................................................................................................................. .................................................................................................................................................. .................................................................................................................................................. [Total: 5] [5]