DEPARTMENT OF CHEMICAL TECHNOLOGY PART ONE
advertisement

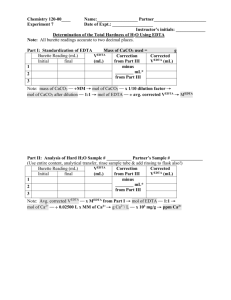
MIDLANDS STATE UNIVERSITY DEPARTMENT OF CHEMICAL TECHNOLOGY DEPARTMENT OF CHEMICAL TECHNOLOGY PART ONE ANALYTICAL CHEMISTRY (CT104) EDTA TITRATIONS Ethylenedinitrilotetraacetic acid (EDTA:1,2 diaminoethanetetra-acetic acid) is one of a class of aminopolycarboxylic acids which form soluable strong complexes with many metal ions. When a solution containing asuitable divalent metal ion is titrated with EDTA , the reaction that occurs can be formulated: M2+ H2Y2 - + MY2- 2H+ + which is a special case of the general equation: Mm+ + HnYn-4 MYm-4 + nH+ Where Y4- is the fully deprotonated anion of EDTA. The free acid has the formula: - OOC-CH2 CH2-COOH N-CH2-CH2-N CH2-COO- HOOC-CH2 It is a hexadentate ligand and one of its most interesting features is that it forms 1:1 complexes with metal ions regardless of their charge. The first practical application of the EDTA titration was the determination of water hardness (calcium and magnesium salts) but it can be used for many other ions as well. In this practical EDTA titrations will be illustrated by using it to titrate zinc, calcium, magnesium and nickel. The end-points of titrations with EDTA are usually detected by metallochromic indicators. These are organic colourship matters that undergo a colour change when they form metal complexes. Generally speaking, not only the dyestuff but also its metal complex are coloured, so that the end-point of the titration is not characterized by e appearance or disappearance of a color , but rather a change in color. Two of the most common metallochromic indicators are murexide and eriochrome black T (Erio T for short). Murexide Murexide , which is blue in alkaline solution , forms a pink complex with calcium ions.When a solution containing calcium and murexide is titrated with EDTA, the original blue color of the indicator is restored when all the calcium ions have been complexed .The anion of murexide has the following constitution. HN O C C O C HN C O O C NH N C C O O C NH Several other metal ions such as cadmium , cobalt , copper , mercury , nickel and zinc give a color with murexide. Magnesium is without effect ; hence , at a suitable pH , calcium can be titrated in the presence of magnesium when murexide is used as indicator. Eriochrome Black T This indicator is blue between pH 6.3 and 11.3.The magnesium complex is wine-red ; when magnesium is titrated with EDTA in the presence of this indicator between these pH limits , the latter reverts to its original blue color when all the magnesium ions have been complexed.The optimum pH is 10. The complex formed between calcium and this dye is too unstable for indicator purposes.When a solutuion containing both calcium and magnesium os titrated with EDTA , calcium is preferentially complexed by EDTA , but an end point is not obtained until all the magnesium has also been complexed. Hence the sum of magnesium and calcium is obtained .Several other metals give similar color reactions and can be titrated in the same way as magnesium, e.g. zinc ,cadmium ,lead and manganese. Zinc In this practical EDTA will be standardized with a zinc solution.This titration presents no difficulties and involves titration in an ammoniacal solution with Erio T as indicator. Magnesium The indicator Erio T is used in this titration.The stability of the complexes of Mg with EDTA and with the indicator are just high enough to permit an accurate titration and in point of the fact that color change at the end-point (winered) is less sharp than in many other complexometric titrations. The titration must be continued until the last suggestion of a reddish blue has gone.Interfering metals such as Cu,Ni,Co,Fe etc are masked by KCN - sodium sulphide is equally effective as a precipitant. Calcium The titration of calcium with EDTA can be carried out in very dilute solutions with murexide as indicator. However,the the color change from red to blue- violet which takes place place in strongly alkaline medium (Ph 12) is not so sharp as in metallochromic indicators. Nickel Nickel can be titrated directly in strongly ammoniacal solution using murexide as indicator .With all nickel titrations ,, it is necessary to titrate slowly near the end-point, as the rate of formation of the Ni-complex is not very high. Ni can also be determined by way of back-titrations, and this has the advantage that the lower rate of reaction is now of no consequence.The back-titration can be carried out with either copper or zinc. Procedure Standardization of EDTA solution Weigh accurately about 0.25g of zinc oxide and transfer into a conical flask .Add 0.5M HCl dropwise until all the zinc oxide has dissolved .Cool and transfer to a 250ml volumetric flask with distilled water .Pipette 10ml portions into each of three 250ml conical flasks ;add concentrated ammonia solution in excess to dissolve the precipitate formed .Titrate with the EDTA solution using Erio T indicator .Calculate the molarity of the EDTA solution. HARDNESS OF WATER Hardness of water is due calcium and magnesium salts which are present as hydrogen carbonates (temporary hardness), and sulphates, chlorides and nitrates (permanent hardness). These salts are leached out by natural water, usually containing dissolved CO2, as it flows among rock strata underground. Hard water has poor washing properties and when heated leaves deposits of solid CaCO 3. The determination of water hardness is, therefore, important in many industrial processes where water is an important raw material, so that where necessary, appropriate steps can be taken. DETERMINATION OF Ca HARDNESS IN WATER Pipette 100ml of bore-hole water into a 250ml conical flask, add 2ml of the ammonia buffer pH 10 and add 2 drops of freshly prepared 1% sodium sulphide solution. After 1 minute add a tip of a spatula Erio T indicator and titrate with the standardized EDTA solution until the last traces of red have disappeared from the solution. Calculate the total hardness as ppm CaCO3. DETERMINATION OF NICKEL Pipette 100ml of the neutral sample solution into a 250ml conical flask. Add murexide indicator. Then add 10 ml of 1M NH4Cl. If the pH of the solution is below 7 the indicator will have an orange-yellow colour (due to NiH4D+) and dilute ammonia must be added dropwise until the colour changes to yellow (due to NiH 2D-). Start the titration and continue until the end-point is near. If the colour reverts to orange because of a fall in pH, add a few drops of ammonia and continue the titration. Just before the end-point make the solution strongly ammonical by adding 10ml of concentrated ammonia and titrate to a brilliant colour change from yellow to bluish-violet. Calculate the concentration of Ni in the sample solution.