Experiment 6 - EDTA Titration of Hardness of Water
advertisement
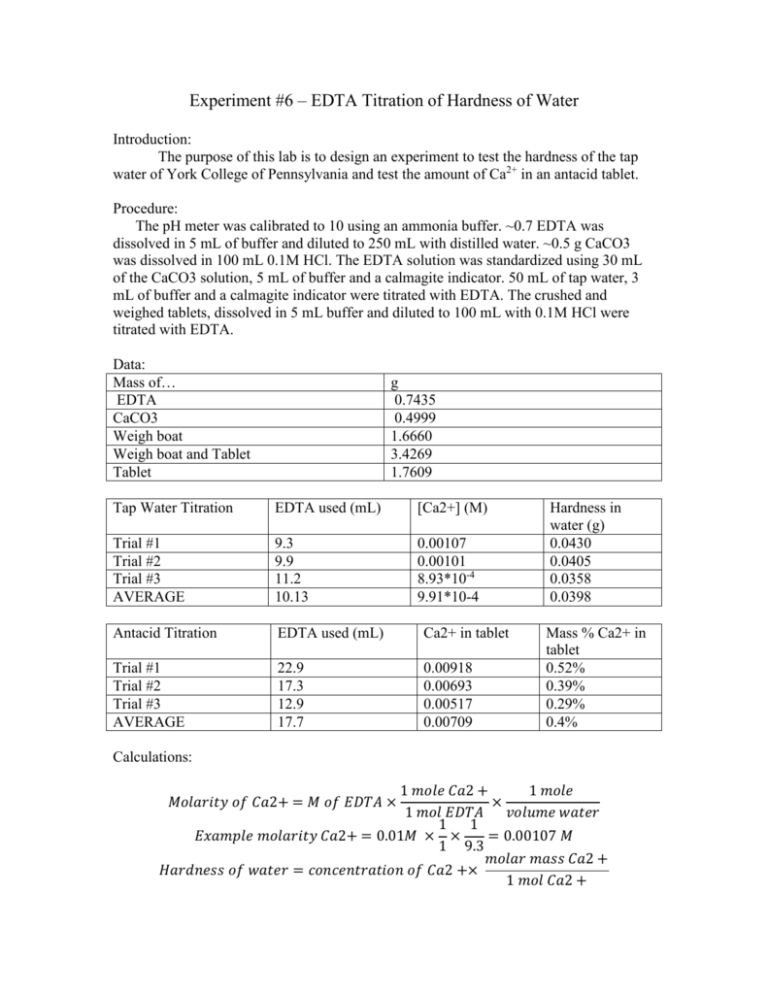
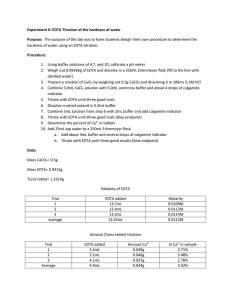
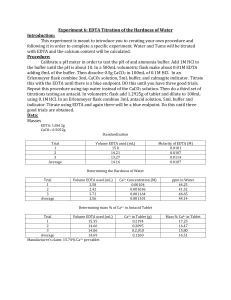
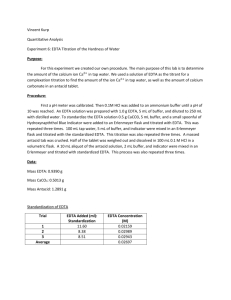
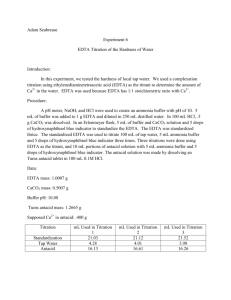
Experiment #6 – EDTA Titration of Hardness of Water Introduction: The purpose of this lab is to design an experiment to test the hardness of the tap water of York College of Pennsylvania and test the amount of Ca2+ in an antacid tablet. Procedure: The pH meter was calibrated to 10 using an ammonia buffer. ~0.7 EDTA was dissolved in 5 mL of buffer and diluted to 250 mL with distilled water. ~0.5 g CaCO3 was dissolved in 100 mL 0.1M HCl. The EDTA solution was standardized using 30 mL of the CaCO3 solution, 5 mL of buffer and a calmagite indicator. 50 mL of tap water, 3 mL of buffer and a calmagite indicator were titrated with EDTA. The crushed and weighed tablets, dissolved in 5 mL buffer and diluted to 100 mL with 0.1M HCl were titrated with EDTA. Data: Mass of… EDTA CaCO3 Weigh boat Weigh boat and Tablet Tablet g 0.7435 0.4999 1.6660 3.4269 1.7609 Tap Water Titration EDTA used (mL) [Ca2+] (M) Trial #1 Trial #2 Trial #3 AVERAGE 9.3 9.9 11.2 10.13 0.00107 0.00101 8.93*10-4 9.91*10-4 Antacid Titration EDTA used (mL) Ca2+ in tablet Trial #1 Trial #2 Trial #3 AVERAGE 22.9 17.3 12.9 17.7 0.00918 0.00693 0.00517 0.00709 Hardness in water (g) 0.0430 0.0405 0.0358 0.0398 Mass % Ca2+ in tablet 0.52% 0.39% 0.29% 0.4% Calculations: 1 𝑚𝑜𝑙𝑒 𝐶𝑎2 + 1 𝑚𝑜𝑙𝑒 × 1 𝑚𝑜𝑙 𝐸𝐷𝑇𝐴 𝑣𝑜𝑙𝑢𝑚𝑒 𝑤𝑎𝑡𝑒𝑟 1 1 𝐸𝑥𝑎𝑚𝑝𝑙𝑒 𝑚𝑜𝑙𝑎𝑟𝑖𝑡𝑦 𝐶𝑎2+ = 0.01𝑀 × × = 0.00107 𝑀 1 9.3 𝑚𝑜𝑙𝑎𝑟 𝑚𝑎𝑠𝑠 𝐶𝑎2 + 𝐻𝑎𝑟𝑑𝑛𝑒𝑠𝑠 𝑜𝑓 𝑤𝑎𝑡𝑒𝑟 = 𝑐𝑜𝑛𝑐𝑒𝑛𝑡𝑟𝑎𝑡𝑖𝑜𝑛 𝑜𝑓 𝐶𝑎2 +× 1 𝑚𝑜𝑙 𝐶𝑎2 + 𝑀𝑜𝑙𝑎𝑟𝑖𝑡𝑦 𝑜𝑓 𝐶𝑎2+ = 𝑀 𝑜𝑓 𝐸𝐷𝑇𝐴 × 40.078𝑔 = 0.043𝑔 1 1 𝑚𝑜𝑙 𝐶𝑎2 + 𝑚𝑜𝑙𝑎𝑟 𝑚𝑎𝑠𝑠 𝐶𝑎2 + 𝑚𝑎𝑠𝑠 𝑜𝑓 𝐶𝑎2 + 𝑖𝑛 𝑡𝑎𝑏𝑙𝑒𝑡𝑠 = 𝑀 𝐸𝐷𝑇𝐴 × × 1 𝑚𝑜𝑙 𝐸𝐷𝑇𝐴 1 𝑚𝑜𝑙 𝐶𝑎2 + 1 40.078𝑔 𝑒𝑥𝑎𝑚𝑝𝑙𝑒 𝑜𝑓 𝑚𝑎𝑠𝑠 𝑜𝑓 𝐶𝑎2+: 0.1𝑀 × × = 0.00918𝑔 1 1 𝑚𝑎𝑠𝑠 𝑜𝑓 𝐶𝑎2 + % 𝑖𝑛 𝑒𝑎𝑐ℎ 𝑡𝑎𝑏𝑙𝑒𝑡 = × 100 𝑚𝑎𝑠𝑠 𝑜𝑓 𝑡𝑎𝑏𝑙𝑒𝑡 0.00918 𝑒𝑥𝑎𝑚𝑝𝑙𝑒 𝑜𝑓 %: × 100 = 0.52% 1.7609 𝑒𝑥𝑎𝑚𝑝𝑙𝑒 𝑜𝑓 ℎ𝑎𝑟𝑑𝑛𝑒𝑠𝑠 𝑜𝑓 𝑤𝑎𝑡𝑒𝑟: 0.00107𝑀 × Conclusions: My partner and I determined the procedure by analyzing past experiments and their successes. After we attempted the experiment using the first procedure, the results didn’t come through as expected and we searched for a new procedure. The second procedure would much better and the results proved correct in regards to our assumptions. Although we did not record the expected calcium included in the tablet, the manufacturer’s claims had to have been higher than what we calculated. Sources of error could have included over-titration and incorrect measurement of mass of reagents. These are human error and could have been avoided with a little more caution.