Chem 108 Supplementary Problems
advertisement

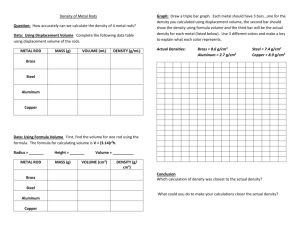
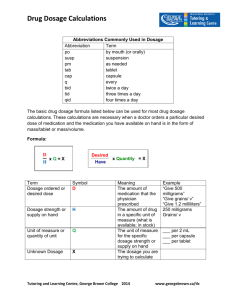
Chem 108 Supplementary Problems - Chapter 2 1. How many significant figures are there in the following measurements? a. 200 km b. 0.002 m c. 0.030 ft d. 0.00506 in e. 20. m f. 20.0 m 2. A student determines the mass of an object using a balance reading 0.10 g. In his lab report, the student records the mass as 0.1 g. Why is this wrong? 3. Perform the following conversions. Show the correct number of significant figures in your answers! a. 22 mi/h = _____ km/h b. 35 mi/h = _____ ft/s c. 3434 cm3 = _____ mL 2 d. 0.58 m = _____ cm2 3 e. 8900 cm = _____ m3 f. 25 mg/mL = ____ g/L g. 25.0 mg/mL = ____ g/mL h. 1.42 g/cm2 = ____ mg/mm2 4. A swimming pool has a volume of 15000 gallons. How many hours will it take to fill the pool if water is added at a rate of 5.0 gal/min? 5. The required dosage for a certain medication is 4.5 mg per kg of body mass. a. What mass of medication is necessary for female weighing 124 pounds? b. If the medication is available in capsules which contain 50. mg of medication, how many capsules are required for the correct dosage? 6. What is the volume in mL of a piece of gold that weighs 0.44 lb? The density of gold is 19.32 g/cm3. 7. What is the mass in kg of a piece of gold having a volume of 2.7 x 10-4 m3? 8. An experiment is performed to determine the density of a metal. When a 24.8 g piece of the metal is placed in a graduate cylinder containing 32.4 mL of water, the volume increases to 38.6 mL. What is the density of the metal? 9. What volume of a container (in liters) would be needed to hold 7.2 kg of gasoline? The density of gasoline is 0.68 g/mL. 10. A 5.00 kg sample of bituminous coal contains 3.2 kg of carbon and 0.500 kg of ash. How many kg of ash are in 425 of this coal? Answers 1a: 1, b: 1, c: 2, d: 3, e: 2, f:3; 3a: 35, b: 51, c: 3434, d: 5800, e: 0.0089, f: 25, g: 0.00250; h: 14.2 4: 50. h; 5a: 250 mg, b: 5.0 capsules; 6: 10. cm3; 7: 5200 g; 8: 4.0 g/cm3; 9: 11 L; 10: 42.5 kg