New 21st Century Chemistry
Suggested answers to in-text activities and unit-end exercises
Topic 6 Unit 24
In-text activities
Checkpoint (page 38)
1
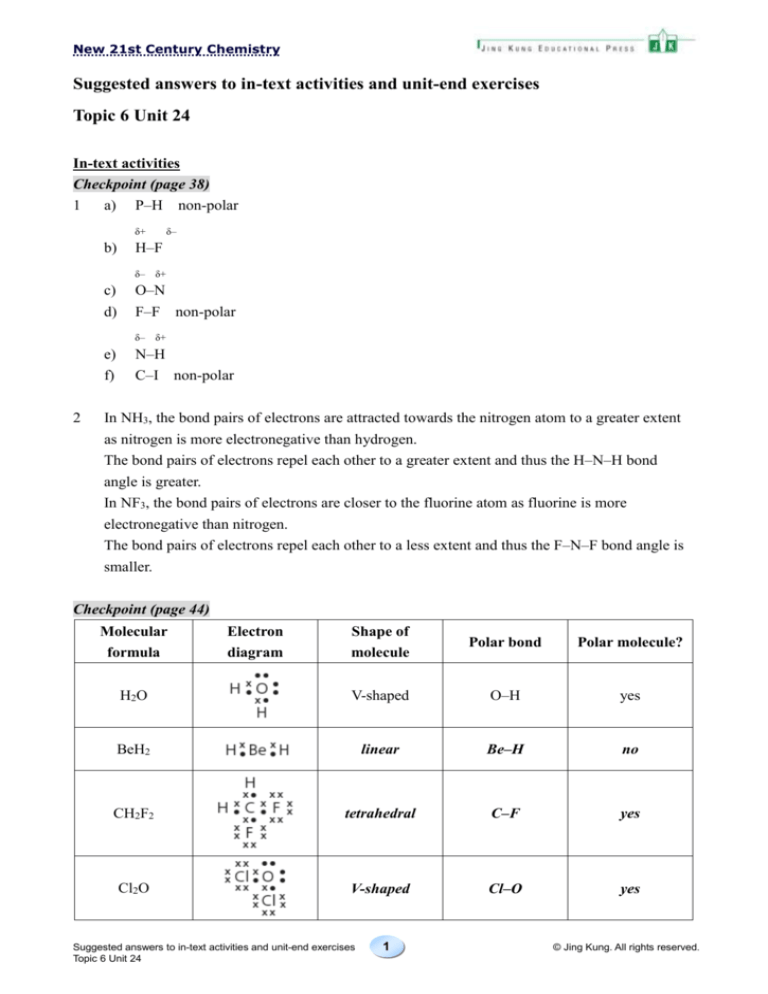
a) P–H non-polar
δ+
b)
δ–
H–F
δ– δ+
c)
O–N
d)
F–F non-polar
δ– δ+
e)
f)
2
N–H
C–I non-polar
In NH3, the bond pairs of electrons are attracted towards the nitrogen atom to a greater extent
as nitrogen is more electronegative than hydrogen.
The bond pairs of electrons repel each other to a greater extent and thus the H–N–H bond
angle is greater.
In NF3, the bond pairs of electrons are closer to the fluorine atom as fluorine is more
electronegative than nitrogen.
The bond pairs of electrons repel each other to a less extent and thus the F–N–F bond angle is
smaller.
Checkpoint (page 44)
Molecular
formula
Electron
diagram
Shape of
molecule
Polar bond
Polar molecule?
H2O
V-shaped
O–H
yes
BeH2
linear
Be–H
no
CH2F2
tetrahedral
C–F
yes
Cl2O
V-shaped
Cl–O
yes
Suggested answers to in-text activities and unit-end exercises
Topic 6 Unit 24
1
© Jing Kung. All rights reserved.
New 21st Century Chemistry
2
a)
b)
In trichloromethane, each C–Cl bond is polar.
The trichloromethane molecule is tetrahedral in shape.
The individual C–Cl bond dipole moments reinforce each other.
The molecule has a net dipole moment and it is polar.
When a positively charged rod is brought close to the jet of trichloromethane, negative
ends of the molecules are attracted towards the rod.
When a negatively rod is brought close to the jet of trichloromethane, positive ends of the
molecules are attracted towards the rod.
Checkpoint (page 52)
1
Type of attractions
Substance
Instantaneous
dipole-induced
dipole attractions
a) Bromine
✔
b) Liquid sulphur dioxide
✔
c) Methane
✔
Suggested answers to in-text activities and unit-end exercises
Topic 6 Unit 24
2
Permanent
dipole-permanent
dipole attractions
✔
© Jing Kung. All rights reserved.
New 21st Century Chemistry
2
The boiling point of H2S is higher than that of SiH4.
The boiling point of a compound depends on the strength of its intermolecular attractions.
H2S is a polar substance. There are permanent dipole-permanent dipole attractions and
instantaneous dipole-induced dipole attractions between H2S molecules.
SiH4 is a non-polar substance. There are only instantaneous dipole-induced dipole attractions
between SiH4 molecules.
More heat is needed to separate the H2S molecules during boiling.
Checkpoint (page 54)
The boiling point of a compound depends on the strength of its intermolecular attractions.
The intermolecular attractions in the carbon compounds are van der Waals’ forces.
The number of electrons in the molecule / the molecular mass increases from methane to propane.
Hence the strength of van der Waals’ forces also increases from methane to propane. This suggests
that the boiling points should increase from methane to propane in accordance with the data.
Discussion (page 60)
Without hydrogen bonding, the boiling point of H2O would be around –68 °C while that of HF
would be around –88 °C.
HF molecules can form an average of one hydrogen bond per molecule while H2O molecules can
form an average of two hydrogen bonds per molecule.
Hence the difference between the actual boiling point and estimated boiling point without hydrogen
bonding for H2O is much greater than that for HF.
Checkpoint (page 62)
1 a)
Type of attractions
Liquid
Instantaneous
dipole-induced
dipole attractions
Permanent
dipole-permanent
dipole attractions
Hydrogen
bonds
✔
✔
a)
✔
b)
✔
Suggested answers to in-text activities and unit-end exercises
Topic 6 Unit 24
3
© Jing Kung. All rights reserved.
New 21st Century Chemistry
Type of attractions
Liquid
Instantaneous
dipole-induced
dipole attractions
Permanent
dipole-permanent
dipole attractions
✔
✔
Hydrogen
bonds
c)
b)
The boiling point of a compound depends on the strength of its intermolecular attractions.
The boiling point of
is the lowest.
Only weak instantaneous dipole-induced dipole attractions exist between the molecules.
The boiling point of
is the highest.
Hydrogen bonds exist between the molecules.
2
(For the sake of clarity, interaction is not shown at every –OH group.)
Checkpoint (page 69)
1 (a) and (c)
Suggested answers to in-text activities and unit-end exercises
Topic 6 Unit 24
4
© Jing Kung. All rights reserved.
New 21st Century Chemistry
2 a)
Type of attractions
Compound
Instantaneous
dipole-induced
dipole attractions
Permanent
dipole-permanent
dipole attractions
Hydrogen bonds
i)
NH3
✔
✔
✔
ii)
CH3OH
✔
✔
✔
iii)
✔
✔
iv)
✔
b)
Library Search & Presentation (page71)
Proteins
Biological functions of proteins
Proteins are large molecules that occur in every living organism. They are of many types and have
many biological functions. The following table lists some biological functions of proteins.
Some biological functions of proteins
Type
Function
Example
Enzymes
catalyze biological processes
pepsin
Hormones
regulate body processes
insulin
Storage proteins
store nutrients
ferritin
Transport proteins
transport oxygen and other substances
through the body
hemoglobin
Structural proteins
form an organism’s structure
collagen
Protective proteins
help fight infection
antibodies
Contractile proteins
form muscles
actin, myosin
Toxic proteins
serve as a defense for the plant or animal
snake venoms
Suggested answers to in-text activities and unit-end exercises
Topic 6 Unit 24
5
© Jing Kung. All rights reserved.
New 21st Century Chemistry
Amino acids making up the body’s proteins
All proteins are made up of many amino acid units linked together in a long chain. Twenty different
amino acids are used to make the body’s proteins.
An amino acid contains both an amino group and a carboxyl group.
The following table lists some amino acids essential to living organisms.
Name
Abbreviations
Alanine
Ala
Aspartic acid
Asp
Cysteine
Cys
Glycine
Gly
Histidine
His
Serine
Ser
Structure
(shaded portion is the R group of the amino acid)
Suggested answers to in-text activities and unit-end exercises
Topic 6 Unit 24
6
© Jing Kung. All rights reserved.
New 21st Century Chemistry
Name
Abbreviations
Valine
Val
Structure
(shaded portion is the R group of the amino acid)
Peptide link formation
Two amino acids can undergo a condensation reaction to form a dipeptide. A water molecule is
eliminated between the –NH2 group of one amino acid and the –COOH group of the other. The
amino acid units are linked by a peptide link.
From a dipeptide, a tripeptide can be made by adding another amino acid molecule. Peptides that
contain many amino acid units are polypeptides. Proteins are polypeptides consisting of one or
more polypeptide chains.
Levels of protein structure
Chemists usually speak about four levels of structure when describing proteins.
Primary structure
The primary structure specifies the unique amino acid sequence of the polypeptide chain.
Secondary structure
Most polypeptide chains fold in such a way that the segments of the chain orient into regular
patterns, called secondary structures. There are two common kinds of patterns: the α-helix and the
β-pleated sheet.
Suggested answers to in-text activities and unit-end exercises
Topic 6 Unit 24
7
© Jing Kung. All rights reserved.
New 21st Century Chemistry
In the α-helix, the polypeptide chain is coiled tightly in the fashion of a spring. The helix is
stabilized by hydrogen bonds between the N–H group of one amino acid unit and the C=O group on
the 4th amino acid unit away from it.
The following figure shows the α-helical secondary structure of keratin, a fibrous protein found in
wool, hair, fingernails and feathers.
The following figure shows the β-pleated sheet secondary structure found in fibroin, the fibrous
protein found in milk. A polypeptide chain doubles back on itself after a hairpin bend. The two
sections of the chain on either side of the bend line up in a parallel arrangement held together by
hydrogen bonds.
Suggested answers to in-text activities and unit-end exercises
Topic 6 Unit 24
8
© Jing Kung. All rights reserved.
New 21st Century Chemistry
Tertiary structure
Secondary protein structures result primarily from hydrogen bonding between peptide links along
the protein backbone, but higher levels of structure result primarily from interactions of R groups in
the protein.
The tertiary structure of a protein is its three-dimensional shape that arises from further foldings of
its polypeptide chains, foldings superimposed on the coils of the α-helices.
Various forces are involved in stabilizing tertiary structures, including van der Waals’ forces, ionic
linkages, hydrogen bonds and disulphide bridges (refer to the following figure for details).
Quaternary structure
A protein molecule may be made up of more than one polypeptide chain. The overall arrangement
of the polypeptide chains is called the quaternary structure. A variety of interactions including
hydrogen bonding hold the various chains into a particular geometry.
Suggested answers to in-text activities and unit-end exercises
Topic 6 Unit 24
9
© Jing Kung. All rights reserved.
New 21st Century Chemistry
There are two major categories of proteins with quaternary structure — fibrous and globular.
Collagen is a fibrous protein in tendons and muscles, consisting of intertwining polypeptide chains.
Globular proteins are mostly clumped into a shape of a ball. For example, the hemoglobin molecule
consists of four separate polypeptide chains or subunits. These subunits are held together by van der
Waals’ forces and ionic forces.
DNA
Functions of DNA
The nucleic acids are informational molecules because their primary structure contains a code or set
of directions by which they can duplicate themselves and guide the synthesis of proteins. There are
two types of nucleic acids which are polymers found in all living cells. Deoxyribonucleic acid
(DNA) is found mainly in the nucleus of the cell, while ribonucleic acid (RNA) is found mainly in
the cytoplasm of the cell.
Suggested answers to in-text activities and unit-end exercises
Topic 6 Unit 24
10
© Jing Kung. All rights reserved.
New 21st Century Chemistry
Coded in an organism’s DNA is all the information that determines the nature of the organism and
all the directions that are needed for producing the thousands of different proteins required by the
organism.
Structure of nucleic acids
Nucleic acids are polymers made up of nucleotide units linked together to form a long chain. Each
nucleotide is composed of a nucleoside plus phosphoric acid, and each nucleoside is composed of a
sugar plus an amine base.
The sugar in DNA is 2-deoxyribose.
Four different cyclic amine bases occur in DNA: adenine, guanine, cytosine and thymine.
In DNA, the cyclic amine base is bonded to C1’ of the sugar, and the phosphoric acid is bonded to
the C5’ sugar position. The following figure shows the general structures of a nucleoside and a
nucleotide.
Suggested answers to in-text activities and unit-end exercises
Topic 6 Unit 24
11
© Jing Kung. All rights reserved.
New 21st Century Chemistry
Nucleotides join together in nuclei acids by forming a phosphate ester bond between the phosphate
group at the 5’ end of one nucleotide and the hydroxyl group on the sugar component at the 3’ end
of another nucleotide.
This makes the nuclei acid a long unbranched chain with a backbone of sugar and phosphate units
with bases protruding from the chains at regular intervals.
Suggested answers to in-text activities and unit-end exercises
Topic 6 Unit 24
12
© Jing Kung. All rights reserved.
New 21st Century Chemistry
The following diagram shows a segment of one DNA chain. See how the phosphate ester groups
link the 3’- and 5’- OH groups of the sugar units.
Base pairing in DNA: the Watson-Crick Model
According to the model, DNA consists of two polynucleotide strands coiled around each other in a
double helix. The two strands run in opposite directions and are held together by hydrogen bonds
between pairs of bases. Adenine (A) and thymine (T) form two strong hydrogen bonds to each other,
but not to guanine (G) or cytosine (C); G and C form three strong hydrogen bonds to each other, but
not to A or T.
Suggested answers to in-text activities and unit-end exercises
Topic 6 Unit 24
13
© Jing Kung. All rights reserved.
New 21st Century Chemistry
The specific base pairing also means that the two chains of DNA are complementary. Wherever
adenine appears in one chain, thymine must appear opposite it in the other; wherever cytosine
appears in one chain, guanine must appear in the other (refer to the following diagram of the DNA
double helix showing complementary base pairing).
Replication of DNA
Just prior to cell division the double strand of DNA begins to unwind. Complementary strands are
formed along each chain. Each chain acts as a template for the formation of its complement. When
unwinding and duplication are complete, there are two identical DNA molecules where only one
had existed before. These two molecules can then be passed on, one to each daughter cell.
Suggested answers to in-text activities and unit-end exercises
Topic 6 Unit 24
14
© Jing Kung. All rights reserved.
New 21st Century Chemistry
Suggested answers to in-text activities and unit-end exercises
Topic 6 Unit 24
15
© Jing Kung. All rights reserved.
New 21st Century Chemistry
Unit-end exercises (pages 75-84)
Answers for the HKCEE (Paper 1) and HKALE questions are not provided.
1
2
a)
The electronegativity of an element represents the power of an atom of that element to
attract a bonding pair of electrons towards itself in a molecule.
b)
δ– δ+
c)
C–H C=O O–H N–H
CH4
δ+
δ–
δ– δ+ δ– δ+
Suggested answers to in-text activities and unit-end exercises
Topic 6 Unit 24
16
© Jing Kung. All rights reserved.
New 21st Century Chemistry
3
a)
b)
c)
The electronegativity values of carbon and chlorine determine where the partial charges
are placed on the molecule.
Yes
Each C–Cl bond is polar.
Because of its tetrahedral shape, the individual C–Cl bond dipole moments reinforce each
other.
Hence the whole molecule has a net dipole moment.
4 a)
Substance
Boiling point (K)
Type(s) of intermolecular forces
Propane
229
instantaneous dipole-induced dipole attractions
338
instantaneous dipole-induced dipole attractions,
permanent dipole-permanent dipole attractions,
hydrogen bonds
Methanol
b)
The boiling point of a compound depends on the strength of its intermolecular attractions.
Hydrogen bonds exist between methanol molecules, in addition to permanent
dipole-permanent dipole attractions and instantaneous dipole-induced dipole attractions.
There are only weak instantaneous dipole-induced dipole attractions between propane
molecules.
Hence the boiling point of methanol is higher than that of propane.
5
The volatility of a halogen depends on the strength of its intermolecular attractions.
Van der Waals’ forces exist between halogen molecules.
The number of electrons in the molecule increases from chlorine to iodine. Hence the strength
of van der Waals’ forces also increases from chlorine to iodine.
Thus the trend in volatility of the three halogens is chlorine > bromine > iodine.
6
B
Van der waals’ forces exist in methane and neon.
The stronger van der Waals’ forces in methane (due to greater molecular surface area
allowing greater contact between molecules) account for its higher boiling point as more
heat is required to separate its molecules during boiling.
7
C
Although the H–Cl bond is polar, the chlorine atom is quite large and its lone pairs of
electrons are not very accessible to a hydrogen atom.
Hence there is no strong attraction between the hydrogen atom and the lone pair on the
chlorine atom of another HCl molecule.
Suggested answers to in-text activities and unit-end exercises
Topic 6 Unit 24
17
© Jing Kung. All rights reserved.
New 21st Century Chemistry
A HCl molecule will not form a hydrogen bond with another HCl molecule.
8
D
X is non-polar. It does not mix with water due to the difference in the strength of
intermolecular attractions between water molecules and those between molecules of X.
Thus, X is insoluble in water.
Y is soluble in water because hydrogen bonds can form between molecules of Y and
water molecules.
The water solubility of Z is higher than that of Y.
Each molecule of Z has two –OH groups that can take part in hydrogen bonding while
each molecule of Y has only one –OH group that can take part in hydrogen bonding.
9
D
(1) In a BF3 molecule, each B–F bond is polar.
A BF3 molecule has a trigonal planar shape. The three identical bond dipole
moments cancel one another out exactly.
So a BF3 molecule is non-polar.
(2) In a CCl3Br molecule, all the bonds are polar.
The CCl3Br molecule is tetrahedral in shape. The individual bond dipole moments
do not cancel one another out exactly.
The molecule has a net dipole moment and it is polar.
(3) In a NF3 molecule, each N–F bond is polar.
A NF3 molecule has a trigonal pyramidal shape.
Suggested answers to in-text activities and unit-end exercises
Topic 6 Unit 24
18
© Jing Kung. All rights reserved.
New 21st Century Chemistry
The individual N–F bond dipole moments reinforce each other.
The NF3 molecule has a net dipole moment and it is polar.
10
B
(1) In a hexane molecule, each C–H bond can be regarded as non-polar. Thus hexane is
a non-polar liquid.
A stream of hexane would NOT be deflected by the charged rod.
(2) In a trichloromethane molecule, each C–Cl bond is polar.
The trichloromethane molecule is tetrahedral in shape. The individual C–Cl bond
dipole moments reinforce each other.
The molecule has a net dipole moment and thus trichloromethane is a polar liquid.
A stream of trichloromethane would be deflected by the charged rod.
(3) In a tetrachloromethane molecule, each C–Cl bond is polar.
A tetrachloromethane molecule has a tetrahedral shape. The four identical bond
dipole moments cancel one another out exactly.
As a result, the molecule has no net dipole moment. Thus tetrachloromethane is a
non-polar liquid.
Suggested answers to in-text activities and unit-end exercises
Topic 6 Unit 24
19
© Jing Kung. All rights reserved.
New 21st Century Chemistry
A stream of tetrachloromethane would NOT be deflected by the charged rod.
11
12
a)
The electronegativity of an element represents the power of an atom of that element to
attract a bonding pair of electrons towards itself in a molecule.
b)
A BF3 molecule has a trigonal planar shape.
The three identical bond dipole moments cancel one another out exactly.
As a result, the molecule has no net dipole moment.
a)
In a propanone molecule, the C=O bond is polar.
The molecule has a net dipole moment and thus propanone is a polar molecule.
b)
i)
The jet of propanone is deflected by the positively charged rod.
The negative ends of the molecules are attracted towards the rod.
Suggested answers to in-text activities and unit-end exercises
Topic 6 Unit 24
20
© Jing Kung. All rights reserved.
New 21st Century Chemistry
ii)
The jet of propanone is deflected by the negatively charged rod.
The positive ends of the molecules are attracted towards the rod.
13
a)
PF3
b)
PF3 – trigonal pyramidal shape
PF5 – trigonal bipyramidal shape
c)
Each P–F bond is polar.
A PF3 molecule has a trigonal pyramidal shape. The individual polar P–F bond dipole
moments reinforce each other.
The PF3 molecule has a net dipole moment and it is polar.
NF5 do not exist.
Nitrogen cannot form compounds with more than 8 electrons in the outermost shell of its
atom.
d)
14
—
15
a)
i)
ii)
PF5
Electron pairs repel one another and stay as far apart as possible.
A water molecule has two lone pairs and two bond pairs of electrons in the outermost
shell of the oxygen atom.
The four pairs of electrons in the molecule will adopt a tetrahedral arrangement.
As the shape of a molecule is determined only by the arrangement of atoms, thus the
water molecule is V-shaped.
Lone pair-lone pair repulsion is stronger than lone pair-bond pair repulsion, while
lone pair-bond pair repulsion is stronger than bond pair-bond pair repulsion.
Suggested answers to in-text activities and unit-end exercises
Topic 6 Unit 24
21
© Jing Kung. All rights reserved.
New 21st Century Chemistry
Therefore the two lone pairs will stay the furthest apart, and the separation between a
lone pair and a bond pair will be greater than that between two bond pairs.
As a result, the H–O–H bond angle in a water molecule is decreased to 104.5°.
b)
i)
ii) The electronegativity values of boron and chlorine are different.
iii) A BCl3 molecule has a trigonal planar shape.
The three identical B–Cl bond dipole moments cancel one another out exactly.
As a result, the molecule has no net dipole moment. So the molecule is non-polar.
16
a)
b)
c)
The electronegativity value of hydrogen is greater than that of silicon. So in a SiH4
molecule, each Si–H bond is polar.
A SiH4 molecule has a tetrahedral shape. The four identical Si–H bond dipole moments
cancel one another out exactly.
As a result, the molecule has no net dipole moment. So the SiH4 molecule is non-polar.
The electronegativity value of sulphur is greater than that of hydrogen. So in a H2S
molecule, each S–H bond is polar.
Because of the V-shape of the H2S molecule, the individual S–H bond dipole moments
reinforce each other.
The H2S molecule has a net dipole moment and it is polar.
The boiling point of a compound depends on the strength of its intermolecular attractions.
There are permanent dipole-permanent dipole attractions and instantaneous
dipole-induced dipole attractions between H2S molecules while there are only
instantaneous dipole-induced dipole attractions between SiH4 molecules.
Hence more heat is needed to separate the H2S molecules than the SiH4 molecules during
boiling.
17
a)
The boiling point of a compound depends on the strength of its intermolecular attractions.
The intermolecular attractions in both chloroethane and 1-chloroprapane are van der
Waals’ forces.
A 1-chloropropane molecule contains more electrons than a chloroethane molecule.
Suggested answers to in-text activities and unit-end exercises
Topic 6 Unit 24
22
© Jing Kung. All rights reserved.
New 21st Century Chemistry
So the van der Waals’ forces between 1-chloropropane molecules are stronger than that
between chloroethane molecules. More heat is need to separate the 1-chloropropane
b)
18
—
19
a)
b)
molecules during boiling.
The shape of a 1-chloropropane molecule is more spread-out while that of a
2-chloropropane molecule is more compact.
This allows greater surface contact between 1-chloropropane molecules than between
2-chloropropane molecules.
Hence the van der Waals’ forces between 1-chloropropane molecules are greater than
those between 2-chloropropane molecules. More heat is needed to separate the
1-chloropropane molecules during boiling.
Hydrogen bonds exist between ethanol molecules in addition to permanent
dipole-permanent dipole attractions and instantaneous dipole-induced dipole attractions.
There are only instantaneous dipole-induced dipole attractions between
tetrachloromethane molecules.
A liquid with strong intermolecular forces has a higher viscosity than one with weak
intermolecular forces.
Hence the viscosity of ethanol is higher than that of tetrachloromethane.
Both ethanol and glycerol molecules can form hydrogen bonds.
Each glycerol molecule has three –OH groups that can take part in hydrogen bonding
while each ethanol moleucle has only one –OH group.
Each glycol molecule can form more hydrogen bonds.
Furthermore, because of their shape, the glycerol molecules tend to become entangled
rather than to slide past one another. These factors contribute to the high viscosity of
glycerol.
20
a)
i)
ii)
Hydrogen bond
b)
Ammonia molecules can form hydrogen bonds with water molecules while phosphine
molecules cannot.
Suggested answers to in-text activities and unit-end exercises
Topic 6 Unit 24
23
© Jing Kung. All rights reserved.
New 21st Century Chemistry
21
—
22
a)
i)
ii)
The boiling point of a compound depends on the strength of its intermolecular
attractions.
Only weak van der Waals’ forces exist between methane molecules while there are
hydrogen bonds and van der Waals’ forces between ammonia molecules.
More heat is needed to separate the ammonia molecules than the methane molecules
during
boiling. Hence the boiling point of ammonia is higher than that of methane.
The electronegativity value of oxygen is greater than that of nitrogen, making the
O–H bonds more polar than the N–H bonds. Hence the hydrogen bonds in water are
stronger than those in ammonia.
Furthermore, the oxygen atom in each water molecule has 2 lone pairs of electrons
for hydrogen bonding while the nitrogen atom in each ammonia molecule has only 1
lone pair of electrons for hydrogen bonding. Hence each water molecule can take
part in hydrogen bonding to twice the extent.
More heat is needed to separate the water molecules than the ammonia molecules
during boiling.
Hence the boiling point of water is higher than that of ammonia.
iii) The intermolecular attraction in both methane and silane are van der Waals’ forces.
b)
i)
ii)
A silane molecule contains more electrons than a methane molecule.
So the van der Waals’ forces between silane molecules are stronger than those
between methane molecules.
More heat is needed to separate the silane molecules than the methane molecules
during boiling.
Hence the boiling point of silane is higher than that of methane.
Hydrogen bonding exists in hydrogen fluoride. Hence its boiling point should be
high.
As fluorine is more electronegative than nitrogen, the hydrogen bonds in hydrogen
fluoride should be stronger than those in ammonia.
Hence the boiling point of hydrogen fluoride should be higher than that of ammonia.
There is 1 hydrogen atom per hydrogen fluoride molecule for hydrogen bonding
while there are 2 hydrogen atoms per water molecule for hydrogen bonding. Thus
the hydrogen bonding in hydrogen fluoride should be less extensive.
Hence the boiling point of hydrogen fluoride should be lower than that of water.
The intermolecular attractions in both silane and germanium hydride are van der
Waals’ forces.
A germanium hydride molecule contains more electrons than a silane molecule.
Suggested answers to in-text activities and unit-end exercises
Topic 6 Unit 24
24
© Jing Kung. All rights reserved.
New 21st Century Chemistry
So the van der Waals’ forces between germanium hydride molecules should be
stronger than those between silane molecules.
More heat should be needed to separate the germanium hydride molecules than the
silane molecules during boiling. Hence the boiling point of germanium hydride
should be higher than that of silane.
23
a)
b)
c)
d)
A large dipole between a hydrogen atom and a highly electronegative oxygen atom
A lone pair of electrons on another oxygen atom, with which the partially positively
charged hydrogen atom can line up
Permanent dipole-permanent dipole attractions
i) The boiling point of a compound depends on the strength of its intermolecular
attractions.
Only van der Waals’ forces exist between chloromethane molecules
while there are hydrogen bonds and van der Waals’ forces between water molecules.
ii)
24
a)
b)
More heat is needed to separate the water molecules than the chloromethane
molecules during boiling.
The intermolecular attractions in both chloromethane and bromomethane are van der
Waals’ forces.
A bromomethane molecule contains more electrons than a chloromethane molecule.
So the van der Waals’ forces between bromomethane molecules are stronger than
those between chloromethane molecules.
More heat is needed to separate the bromomethane molecules than the
chloromethane molecules during boiling.
Evaporation is a process that takes in heat. It can cause a temperature decrease in the
surroundings.
Other factors being equal, the higher the rate at which molecules leave the liquid surface,
the more extreme the temperature in the surroundings drops.
As the temperature change for pentane is greater than that for butan-1-ol, it can be
deduced that pentane evaporates faster than butan-1-ol.
Only weak van der Waals’ forces exist between pentane molecules
while there are hydrogen bonds and van der Waals’ forces between butan-1-ol molecules.
Thus pentane molecules can break away from the rest of the liquid more easily.
Suggested answers to in-text activities and unit-end exercises
Topic 6 Unit 24
25
© Jing Kung. All rights reserved.