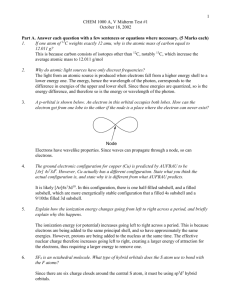
The decomposition reaction pathways of BCl3+CH4+H2 in the
advertisement

Supplement 8 Reaction paths of less favorable decompositions Decomposition of IM48 BC+H+HCl(874.2) TS37(658.8) IM12+HCl(625.3) TS27(571.2) IM44(523.6) TS28(526.3) IM22+Cl(486.9) TS30(597.4) TS1+HCl(588.9) IM29+H(594.4) IM11+HCl IM45(550.6) (566.8) TS26(519.0) IM47(468.4) TS32(424.1) IM46(405.5) TS31(360.4) TS25(309.6) IM9+HCl(492.4) IM10+HCl(482.3) TS29(525.3) IM50(266.8) TS33(205.2) TS64(156.3) IM49(162.7) TS55(59.6) IM22+HCl(80.1) IM48+H(0.0) IM48(0.0) (a) TS75(63.7) TS60(54.9) TS76(106.9) TS56(53.5) TS73(66.2) IM49+H(0.0) IM30+H2(-66.0) IM48+Cl(0.0) IM24+HCl(-14.7) (c) IM24+H2(-18.9) (b) IM29+HCl(24.7) IM49+Cl(0.0) IM23+HCl(-126.3) (d) IM30+HCl(-61.9) (e) Fig. S1 Direct and indirect (via free radical attacking) decompositions of IM48. Data in the parentheses are the relative Gibbs free energies (in kJ·mol-1) at 298.15 K obtained with G3(MP2)//B3PW91. (a) is for the direct decompositions of IM48 (b) is for the paths of IM48 attacked by a hydrogen radical. (c) is for the path of IM48 attacked by a chlorine radical. (d) is for the paths of IM49 attacked by a hydrogen radical. (e) is for the paths of IM49 attacked by a chlorine radical. The paths linked with solid lines are for singlet and those with the dots are for triplet potential energy surfaces. IM48 is the intermediate produced in the lowest barrier path of the direct decompositions of IM74 [Figure 3(a)] and IM64 [Figure 5(a)]. Figure S1(a) shows the isomerization and decomposition paths of IM48. It is found that the isomerization reactions have lower energy barriers and six isomers IM44-IM47, IM49 and IM50 would be produced. IM49 is the isomer of the intra-molecular concerted transposition of the Cl and the H atoms via TS33 with an energy barrier of 205.2 kJ·mol-1. IM46 is the isomer of shifting a H atom via TS32 with the barrier of 424.1 kJ·mol-1. IM50 is the isomer of shifting a Cl atom via TS25 with the barrier of 309.6 kJ·mol-1. IM50 would further transform into IM49 via TS31 by H shift with a barrier of 93.6 kJ·mol-1. The H atom in the -CHCl group of IM49 would shift onto the Cl atom in the same group to produce IM47 via TS29 with a large barrier of 362.6 kJ·mol-1. Either the H atom or the -HCl group attaching the B atom in IM44 would shift onto the C atom to product IM46 or IM47 via TS27 or TS28 with a very small barrier of 47.6 kJ·mol-1 or 2.7 kJ·mol-1. Isomer IM47 would also convert into IM45 by a H shift via two different transition states TS30 and TS37 with the respective energy barriers of 129.0 and 190.4 kJ·mol-1. For the direct dissociations, IM46 would break the B-Cl bond to produce IM12 with an energy of 219.8 kJ·mol-1. IM45 would break the C-Cl bond to produce IM9 by releasing an energy of 58.2 kJ·mol-1. It is notable that the associated transition state was not found. The B-Cl bond in IM48 and the B-H bond in IM49 could also be broken to produce IM22 and IM29, but they need very high energies of 486.9 and 431.7 kJ·mol-1, respectively. IM9 could also be produced by eliminating a HCl molecule via TS26 with an energy barrier of 252.2 kJ·mol-1. IM9 has two different paths to produce the aim product BC. The first is that IM9 will vary its spin from the singlet to the triplet (IM10) state by releasing a small energy of 10.1 kJ·mol-1. IM10 would then decompose directly into BC and a H atom with an energy of 391.9 kJ·mol-1. The second path is that the H atom in IM9 shifts from the C atom onto the B atom via TS1 with a barrier of 96.5 kJ·mol-1 to produce IM11, which would then homogenous break the B-H bond to produce BC with an energy of 307.4 kJ·mol-1. TS26 and IM9 are the species having significant non-dynamic electronic correlations. The MRCISD/6-31G(d) energy barrier 283.6 kJ·mol-1 for TS26 produced from IM50 is comparable with that 252.2 kJ·mol-1 of G3(MP2). The energy difference between TS26 and IM9+HCl is 53.9 kJ·mol-1 and that is 26.6 kJ·mol-1 from G3(MP2). Figure S1(b) is for the decomposition of IM48 attacked by a H radical. A H atom in IM48 can be dissociated to produce IM24 via TS55 with an energy barrier of 59.6 kJ·mol-1 and a Cl atom can also be dissociated to produce IM22 via TS64 with a higher barrier of 156.3 kJ·mol-1. Similarly, Figure S1(c) shows that IM24 can be produced by a Cl radical attacking IM48 via TS75 with a slight higher barrier of 63.7 kJ·mol-1. IM49, the most stable isomer of IM48, would be attacked by either a H and a Cl radical as shown in Figure S1(d) and (e). Figure S1(d) shows that IM49 may decompose into IM30+H2 via TS56 with a lower energy barrier of 53.5 kJ·mol-1, and into IM23+HCl via TS60 with a slightly higher barrier of 54.9 kJ·mol-1. Figure S1(e) shows that a HCl molecule can be released from IM49 via TS73 or TS76 with higher energy barriers of 66.2 or 106.9 kJ·mol-1 associated with a planar IM29 or a linear IM30 product. Decomposition of IM29 TS12(326.9) IM27(304.4) TS16(307.8) IM31(300.4) IM28(283.1) BC+HCl(279.9) TS14(327.2) TS11(57.6) IM29(0.0) IM30(-86.6) Fig. S2 Direct decomposition paths of IM29. Data in the parentheses are the relative Gibbs free energies (in kJ·mol-1) at 298.15 K obtained with G3(MP2)//B3PW91. IM29 is an intermediate that might be produced previously as shown in Figures 5(d), 5(e), 6(b), 7(a), S1(a) and S1(e). Figure S2 is the decomposition paths of IM29. The H atom in the -CHCl group of IM29 could shift onto the B atom to produce IM30 via TS11 with an energy barrier of 57.6 kJ·mol-1 or onto the Cl atom to produce IM31 via TS14 with an energy barrier of 327.2 kJ·mol-1. IM31 will rotate the -ClH group around the C-B bond to produce IM27 via TS16 with a very small energy barrier of 7.4 kJ·mol-1. The -ClH group can also shift from C onto B to produce IM28 via TS12 also with a small barrier of 26.5 kJ·mol-1. The isomers IM27 and IM28 will then automatically decompose directly into BC and a HCl molecule with energies of -24.5 and -3.2 kJ·mol-1, respectively. Decomposition of IM30 and IM32 BC+H+Cl(773.3) IM17+H(615.6) IM11+Cl(465.9) IM15+H(395.4) TS15(111.4) IM32(72.4) IM30(0.0) Fig. S3 Decomposition reaction paths of IM30 and IM32 radicals. Data in the parentheses are the relative Gibbs free energies (in kJ·mol-1) at 298.15 K obtained with G3(MP2)//B3PW91. IM30 is an intermediate that might be produced as shown in Figures S1(d), S1(e) and Figure S2. IM32 is another intermediate that could be produced as shown in Figure 5(b). Figure S3 shows that IM30 and IM32 are practically associated with TS15 by Cl atom shift. The energy barrier is 111.4 kJ·mol-1 to produce IM32. IM32 would decompose the H and then the Cl atom to produce BC. The energies are 323.0 and 377.9 kJ·mol-1. IM30 would also dissociate either a Cl atom or a H atom to produce IM11 or IM17 but the energy is at least 465.9 kJ·mol-1 which predicts the reaction in this channel is less favored although the following dissociation to produce BC needs smaller energy of 307.4 or 157.7 kJ·mol-1. Decomposition of IM22 and IM23 TS4(461.0) TS6(415.8) BC+H2 (383.0) TS5(98.0) IM22(0.0) IM23(-43.7) Fig. S4 Decomposition reaction paths of IM22 and IM23 radicals. Data in the parentheses are the relative Gibbs free energies (in kJ·mol-1) at 298.15 K obtained with G3(MP2)//B3PW91. IM22 and IM23 are the products from the dissociation of IM48 as shown in Figure S1(a), S1(b) or S1(d). Figure S4 shows that IM22 and IM23 would convert into each other via TS5 by a H atom shift. The barrier from IM22 to IM23 is 98.0 kJ·mol-1. IM22 would eliminate a H2 molecule to produce the aim product BC via TS4 with an energy barrier of 461.0 kJ·mol-1. IM23 would also eliminate a H2 molecule to produce the product BC via TS6 with an energy barrier of 459.5 kJ·mol-1. Obviously, these final steps are less feasible due to the high barriers. Decomposition of IM62 and IM63 IM8+IM14(490.6) TS58(424.9) IM38+Cl(388.5) IM7+IM14(449.8) TS57(338.5) IM22+HCl(298.8) IM39+Cl(279.9) IM21+IM3(247.8) IM48+H(218.7) IM24+H2(199.8) TS54(152.3) IM62(32.0) IM63(0.0) Fig. S5 Decomposition reaction paths of IM62 and IM63 radicals. Data in the parentheses are the relative Gibbs free energies (in kJ·mol-1) at 298.15 K obtained with G3(MP2)//B3PW91. The paths linked with solid lines are for singlet and those with the dots are for triplet potential energy surfaces. IM62 and IM63 are the intermediates found in Figure 3. Figure S5 shows that these isomers are associated with TS54 by H shifting and the energy barrier from IM62 to IM63 is 120.3 kJ·mol-1. For all the dissociations, the lowest energy path is that IM62 breaks the C-B bond harmoniously to produce IM21 and IM3 with an energy of 215.8 kJ·mol-1. Competing to this reaction, IM63 can break its B-H bond directly to produce IM48+H with a slightly higher barrier of 218.7 kJ·mol-1. The other reactions have higher energies at least 61.2 kJ·mol-1 more, corresponding to a negligible rate branching ratio of 1.910-11. Decomposition of IM60 IM34+Cl(280.9) TS84(41.5) IM60+H(0.0) IM70(-57.1) IM35+HCl(-64.6) (b) TS89(59.0) TS46(38.9) IM61(8.5) IM60(0.0) (a) IM61+H(0.0) IM34+HCl(-134.4) (c) Fig. S6 Direct and indirect (via free radical attacking) decompositions of IM60. Data in the parentheses are the relative Gibbs free energies (in kJ·mol-1) at 298.15 K obtained with G3(MP2)//B3PW91. (a) is for the direct decomposition of IM60. (b) is for the paths of IM60 attacked by a hydrogen radical. (c) is for the paths of IM61 attacked by a hydrogen radical. Figure S6(a) illustrates the decompositions of IM60 that might be produced by the dissociation of IM80 in Figure 4(a). A Cl atom in the -BCl2 group of IM60 can shift onto the C atom via TS46 to isomerize IM60 into IM61 with a small energy barrier of 38.9 kJ·mol-1. IM61 would then dissociate a Cl atom in the -CCl2 group directly to produce a linear radical IM34(2П) with a higher energy of 272.4 kJ·mol-1. A H radical will attack IM60 to produce IM70 via TS84 with a small barrier of 41.5 kJ·mol-1 as shown in Figure S6(b). IM70 would then easily dissociate into IM35+HCl with a small energy release of 7.5 kJ·mol-1. The MRCI energy barrier for TS84 is slightly larger a value of 43.2 kJ·mol-1. Figure S6(c) shows that IM61 can also be attacked by a H radical to produce IM34+HCl via TS89 with a slightly higher barrier of 59.0 kJ·mol-1. Thus the H attacking reactions are more favorable. The ongoing reactions of IM34 and IM35 will be discussed after the decomposition of IM73 since it will produce an isomer IM36 of IM34 and IM35. Decomposition of IM73 TS85(525.7) IM55+Cl(479.4) IM36+HCl(510.6) IM61+H(421.8) IM60+H(413.3) TS91(411.0) TS90(296.7) IM35+HCl(348.7) TS87(326.5) IM71(225.9) IM72(129.3) IM73(0.0) Fig. S7 Decomposition reaction paths of IM73. Data in the parentheses are the 298.15 K relative Gibbs free energies (in kJ·mol-1) obtained at G3(MP2)//B3PW91 level. IM73 is the favorable product of IM80+H as shown in Figure 4(c). The direct decomposition paths of IM73 are shown in Figure S7. It is found that IM73 could transform into IM72 via TS90 with an energy barrier of 296.7 kJ·mol-1 by intra-molecular concerted transposition of a Cl and the H atoms. The H atom in IM72 would further shift from the -BHCl group onto the -CCl2 group to produce IM71 via TS87 with a barrier of 197.2 kJ·mol-1. IM71 would then decompose a HCl molecule to produce IM36 via TS85 with a larger energy barrier of 299.8 kJ·mol-1. A Cl or H atom in IM72 would be decomposed directly with an energy of 350.1 or 292.5 kJ·mol-1. The reactant in this figure, IM73, would also eliminate a molecular HCl via TS91 with a very high energy barrier of 411.0 kJ·mol-1. Another reaction of IM73 is to dissociate directly the H atom to produce IM60 with an energy of 413.3 kJ·mol-1. All the decompose reactions are less feasible due to the larger energies or energy barriers of about 300 kJ·mol-1. Decomposition of IM34, IM35 and IM36 BC+Cl+Cl(756.6) IM15+Cl(378.7) IM16+Cl(299.2) TS18(169.2) IM36(162.0) TS17(28.9) IM35(0.0) IM34(-61.3) Fig. S8 Decomposition reaction paths of IM34, IM35 and IM36. Data in the parentheses are the relative Gibbs free energies (in kJ·mol-1) at 298.15 K obtained with G3(MP2)//B3PW91. The paths linked with solid lines are for singlet and those with the dots are for triplet potential energy surfaces. IM34, IM35 and IM36 are the radicals produced in the reactions of Figure S6(a), S6(b), S6(c) or Figure S7. The associated reaction paths of these isomers are shown in Figure S8. It is found that both IM35 and IM36 may transform into IM34 via TS17 and TS18 with very small barriers of 28.9 and 7.2 kJ·mol-1, respectively. IM34, the most stable isomer, could dissociate a Cl atom directly to produce IM15 with a larger energy of 360.5 kJ·mol-1. IM15 may then automatically translate into its triplet state IM16 with an energy release of 79.5 kJ·mol-1 and finally decompose the last Cl atom to produce the aim product BC with a large energy of 457.4 kJ·mol-1. Decomposition of IM25 B+H+H+Cl(1108.0) ~ IM1+H+Cl(787.8) ~ IM6+Cl(487.9) TS7(405.3) IM14+H(398.1) IM1+HCl(381.0) TS24(110.5) IM6+HCl(81.4) IM40(97.0) IM25+H(0.0) IM25(0.0) (b) (a) Fig. S9 Decomposition reaction paths of IM25. Data in the parentheses are the relative Gibbs free energies (in kJ·mol-1) at 298.15 K obtained with G3(MP2)//B3PW91. (a) is for the direct decomposition of IM25. (b) is for the paths of IM25 attacked by a hydrogen radical. IM25 (chloroborane BH2Cl) is a B-bearing molecule that could be produced in IM33+H as shown in Figure 9(b). Figure S9(a) shows the decomposition paths of IM25 where all the energies or energy barriers in the first step are at least 398.1 kJ·mol-1. Therefore, the direct decompositions should be infeasible. However, IM25 can be attacked by a H radical to produce a complex IM40 via TS24 with a lower energy barrier of 110.5 kJ·mol-1 as shown in Figure S9(b). The complex will then dissociate into a BH2 radical IM6 and a HCl molecule automatically with an energy release of 15.6 kJ·mol-1. Decomposition of IM59 TS45(37.7) IM58(35.8) IM20+HCl(9.4) IM59(0.0) Fig. S10 Decomposition reaction paths of IM59. Data in the parentheses are the relative Gibbs free energies (in kJ·mol-1) at 298.15 K obtained with G3(MP2)//B3PW91. IM59 is a radical that could be produced in the initial reaction of CH4+BCl3 as shown in Figure 2(a). Figure S10 shows that the H atom in IM59 can easily insert into one of the B-Cl bonds to produce IM58 via TS45 with a small energy barrier of 37.7 kJ·mol-1. IM58 will then dissociate a HCl molecule directly to produce IM20 with an energy release of 26.4 kJ·mol-1. IM20 could also be produced in the reactions shown in Figure 2(a) and Figure 3(a). The ongoing reactions of IM20 have been discussed in Figure 9(b). Decomposition of IM38 and IM39 IM22+H(317.1) TS20(100.2) IM38(0.0) IM39(-108.6) (a) TS47(54.4) TS61(0.4) IM38+Cl(0.0) IM38+H(0.0) IM22+HCl(-89.7) IM22+H2(-93.9) (c) (b) TS48(53.9) IM39+H(0.0) TS59(68.4) TS62(60.4) IM22+HCl(19.0) IM39+Cl(0.0) IM23+HCl(-24.8) IM23+H2(-29.0) (d) (e) Fig. S11 Decomposition reaction paths of IM38 and IM39. Data in the parentheses are the relative Gibbs free energies (in kJ·mol-1) at 298.15 K obtained with G3(MP2)//B3PW91. (a) is for the direct decomposition of IM38 and IM39. (b) is for the path of IM38 attacked by a hydrogen radical. (c) is for the path of IM38 attacked by a chlorine radical. (d) is for the path of IM39 attacked by a hydrogen radical. (e) is for the paths of IM39 attacked by a chlorine radical. IM38 is an intermediate that could be produced in the decomposition of IM62, and IM39 is that of IM63 as shown in Figure S5. Figure S11(a) shows that IM38 could transform into IM39 via TS20 by shifting a H atom in the -CH3 group onto its B atom with an energy barrier of 100.2 kJ·mol-1. The shifted H atom would further be dissociated to produce IM22 with a very high energy of 425.7 kJ·mol-1. Actually, either IM38 or IM39 can be attacked by a H or a Cl radical as shown in Figure S11(b)-(e). A H atom in the -CH3 group of IM38 can be dissociated with very small energy barriers of 54.4 kJ·mol-1 for the H attacking and 0.4 kJ·mol-1 for the Cl attacking as shown in Figure S11(b) and S11(c). A H atom in the -CH2 group of IM39 can also be dissociated with small energy barriers of 53.9 kJ·mol-1 for the H attacking and 68.4 kJ·mol-1 for the Cl attacking as shown in Figure S11(d) and S11(e). Additionally, Figure S11(e) shows that the H atom attaching the B atom of IM39 can be dissociated by Cl attacking via TS62 with a comparable barrier of 60.4 kJ·mol-1. The decomposition paths of IM22 and IM23 have been discussed previously in Figure S4. Decomposition of IM14 B+H+Cl(709.9) IM1+Cl(389.7) IM3+H(223.2) IM14(0.0) Fig. S12 Decomposition reaction paths starting from IM14. Data in the parentheses are the relative Gibbs free energies (in kJ·mol-1) at 298.15 K obtained with G3(MP2)//B3PW91. IM14 is a radical that might be produced in the reactions as shown in Figures 5(c), 9(b), S4 and S9. IM14 would firstly dissociate its H atom or Cl atom to produce IM3 or IM1 with an energy of 223.2 or 389.7 kJ·mol-1. The respective ongoing dissociation to produce the gas phase B atom needs an energy of 486.7 or 320.2 kJ·mol-1. Decomposition of the chlorine substituted hydrocarbons C+H+Cl+H2(1135.2) TS49(98.3) TS52(68.9) TS53(65.4) IM26+H2(-33.9) TS50(-52.5) IM41+H(0.0) IM2+Cl+H2(718.1) CH4+Cl(-87.1) IM5+H+H2(655.6) IM21+HCl(-94.4) (b) TS22(414.6) TS21(399.4) IM8+HCl(371.5) IM42(375.7) IM13+H2(353.7) IM7+HCl(330.8) TS51(26.3) IM41+Cl(0.0) IM41(0.0) IM26+HCl(-29.7) (c) (a) C+H+Cl+H(1169.1) IM2+Cl+H(752.0) ~~ IM5+H+H(689.5) IM8+Cl(401.2) IM13+H(387.6) TS23(23.9) IM26+H(0.0) IM43(7.3) IM8+HCl(-5.6) IM7+HCl(-46.3) IM26(0.0) (d) (e) Fig. S13 Decomposition of the chlorine substituted hydrocarbons. Data in the parentheses are the relative Gibbs free energies (in kJ·mol-1) at 298.15 K obtained with G3(MP2)//B3PW91. (a) is for the direct decompositions of IM41. (b) is for the paths of IM41 attacked by a hydrogen radical. (c) is for the path of IM41 attacked by a chlorine radical. (d) is for the direct decompositions of IM26. (e) is for the paths of IM26 attacked by a hydrogen radical. The paths linked with solid lines are for singlet and those with the dots are for triplet potential energy surfaces. Although this work is mainly focused on the reactions of producing boron and boron carbide, the reactions for the dissociation of the associated hydrocarbons and the chlorine substituted hydrocarbons are expected to be involved for completeness. The discussion on the reactions of the hydrocarbons can be found in one of our previous work [56] with the same theoretical methods. Figure S13(a) shows the direct decompositions of IM41 (chloromethane) that could be produced from either the reaction of the initial reactants [Figure 2(a)] or IM78 [Figure 3(a)]. The lowest energy path is the elimination of a H2 molecule to produce IM13 via TS21 with a barrier of 399.4 kJ·mol-1. IM13 could also be produced in the reactions shown in Figure 5(c). IM13 would firstly dissociate its H atom or Cl atom to produce IM5 or IM2 with an energy of 301.9 or 364.4 kJ·mol-1. The respective ongoing dissociation to produce the gas phase C atom needs an energy of 479.6 or 417.1 kJ·mol-1. The H atom in IM41 could shift onto the Cl atom via TS22 with a higher barrier of 414.6 kJ·mol-1 to produce IM42, which would then dissociate into an excited state of carbene IM8(1A1) and a HCl molecule with a small energy release of 4.2 kJ·mol-1. IM8 will then automatically translate into its ground state IM7(3B1) with an energy release of 40.7 kJ·mol-1. IM7 can be easily dissociated into C by H or CH3 attacking [56]. Figure S13(b) is for the decompositions of IM41 attacked by a H radical. IM41 can dissociate either a Cl atom to produce IM21 via TS53 with a barrier of 65.4 kJ·mol-1 or a H atom to produce IM26 via TS52 with a barrier of 68.9 kJ·mol-1. The Cl atom in IM41 could also be replaced by the attack of a H radical via TS49 with a higher energy barrier of 98.3 kJ·mol-1 to produce one of the initial reactants methane, in which TS49 is a typical structure in the well know SN2 (second-order nucleophilic substitution) reaction. CH4 can be easily attacked by a Cl radical to dissociate a H atom to produce a methyl radical IM21 and a HCl molecule via TS50 with a small energy barrier of 34.6 kJ·mol-1. Figure S13(c) shows the decomposition of chloromethane IM41 attacked by a Cl radical. The decomposition of a H atom to produce chloromethyl radical IM26 and a HCl molecule via TS51 also needs a very small energy barrier of 26.3 kJ·mol-1. Figure S13(d) shows the direct decomposition paths of IM26 where the energies or energy barriers in the first step are at least 387.6 kJ·mol-1. Similar to the reactions of IM25 in Figure S9(b), IM26 can be easily attacked by a H radical to produce a complex IM43 via TS23 with a small barrier of 23.9 kJ·mol-1 as shown Figure S13(e). IM43 would then dissociate into the singlet carbene IM8 and a HCl molecule with an energy release of 12.9 kJ·mol-1.