POLA_26568_sm_SuppInfo
Supporting Information
Statistical Fluorinated Copolymers from Heterogeneous Atom Transfer Radical Copolymerization of Styrene and 2,2,2-Trifluoroethyl Methacrylate with Similar Reactivity Ratios
Ganwei Zhang, Jiwen Hu,
Guping He, Hailiang Zou, Feng Liu, Chengmin Hou, Hongsheng Luo, and
Yuanyuan Tu
Guangzhou Institute of Chemistry, Chinese Academy of Sciences, Guangzhou, P. R. China, 510650
Results
Kinetic study
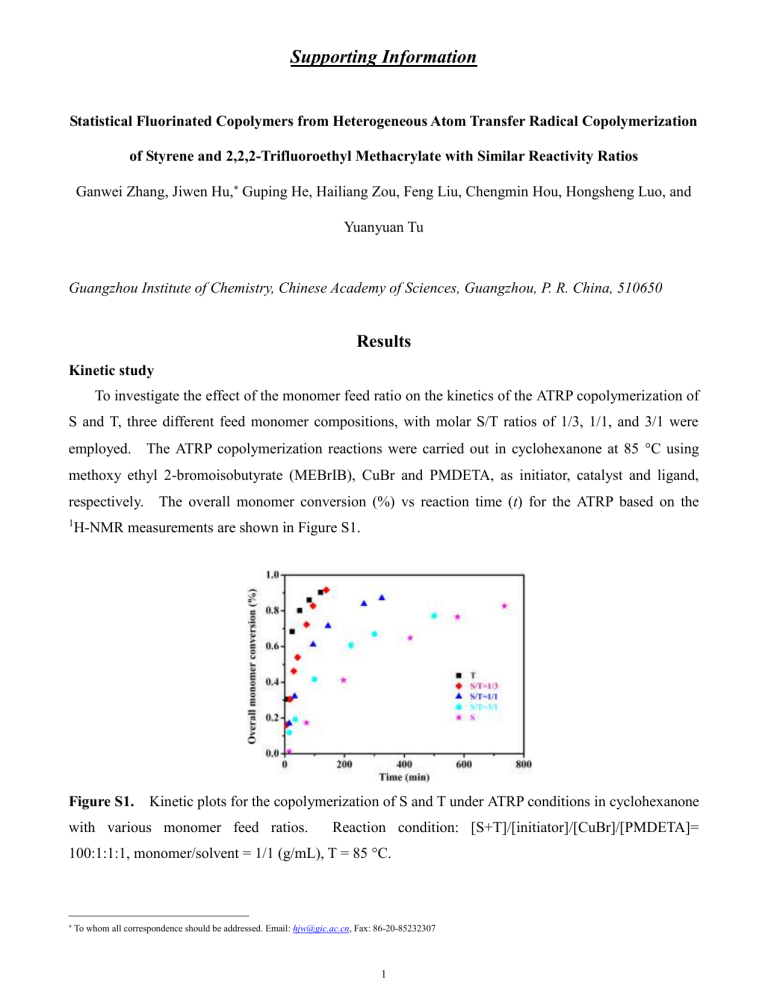
To investigate the effect of the monomer feed ratio on the kinetics of the ATRP copolymerization of
S and T, three different feed monomer compositions, with molar S/T ratios of 1/3, 1/1, and 3/1 were employed. The ATRP copolymerization reactions were carried out in cyclohexanone at 85
C using methoxy ethyl 2-bromoisobutyrate (MEBrIB), CuBr and PMDETA, as initiator, catalyst and ligand, respectively. The overall monomer conversion (%) vs reaction time ( t ) for the ATRP based on the
1 H-NMR measurements are shown in Figure S1.
Figure S1.
Kinetic plots for the copolymerization of S and T under ATRP conditions in cyclohexanone with various monomer feed ratios. Reaction condition: [S+T]/[initiator]/[CuBr]/[PMDETA]=
100:1:1:1, monomer/solvent = 1/1 (g/mL), T = 85 °C.
To whom all correspondence should be addressed. Email: hjw@gic.ac.cn
, Fax: 86-20-85232307
1
UV–Vis Spectroscopic Studies
The UV–visible studies were performed using a UV-1601 spectrometer equipped with
Shimadzu/UV Probe software. Predetermined quantities of the Cu(I)/PMDETA and/or the
Cu(II)/PMDETA catalyst(s), cyclohexanone, styrene (S) or 2,2,2-trifluoroethyl methacrylate (T)
, or methyl methacrylate (MMA), or ethyl methacrylate (EMA) and N,N,N’,N’’,N’’’ -pentamethyl- diethylenetriamine PMDETA were placed in a dried ampoule. Next, the mixtures were dissolved via ultrasonication at 85 0 C. The mixture was subsequently centrifuged and the precipitate was dissolved into cyclohexanone again. This solution was then added to a UV–vis cell, which was placed in the spectrometer for spectral acquisition. The absorbance was measured in the 350–1000 nm range at room temperature. The CuBr
2
/PMDETA solutions were standardized in cyclohexanone by a standard procedure.
Figure S2 shows UV-vis spectra of the Cu(II)/PMDETA in cyclohexanone at various concentrations.
It is apparent that the Cu(II)/PMDETA complex has an absorbance maximum absorption at 790 nm.
The Cu(II)/PMDETA solutions were standardized in cyclohexanone by a standard procedure. As is shown in Figure S2, the solutions of the Cu(II) complexes obeyed Beer’s Law.
Figure S2 . UV-visible spectra of CuBr
2
/PMDETA in cyclohexanone (left) and a plot of the
Absorbance against the concentration of CuBr
2
/PMDETA solutions(right)
A predetermined quantity of the catalyst(s), cyclohexanone, either S, T, MMA, or EMA, and
PMDETA were placed in a dried ampoule. The solubility of Cu(II)/PMDETA was measured using a
UV–vis spectrometer, and the results are shown in Table S1. The monomer T could obviously reduce the Cu(II)/PMDETA solubility in cyclohexanone. In bulk, the absorbance intensity of Cu(II) was very weak in the presence of either T or S, owing to the low solubility of Cu(II)/PMDETA.
Table S1.
Solubility of Cu(II)/PMDETA under various conditions.
2
1
2
3
4
5
6
7
8
9
TFEMA, MMAor
EMA(g)
0.5(T)
------
1.2(T)
1.0(T)
0.6(T)
1.53(MMA)
1.48(EMA)
1.07(MMA)
1.15(EMA)
Styrene
(g)
------
0.5
0.38
0.6
1.12
------
------
0.37
0.35
Cyclohexanone
(mL)
0.5
0.5
1.58
1.6
1.72
1.53
1.48
1.44
1.5
Conditions: [CuBr
2
]/[PMDETA] = 1:2, T = 85°C.
Solubility (mol/ L)
1.19
× 10
-2
1.84
×
10
-2
1.51
× 10
-2
1.69
×
10
-2
1.76
× 10
-2
1.36
×
10
-2
1.37
× 10
-2
1.56
×
10
-2
1.61
× 10
-2
Reactivity Ratio of Monomers
1. At medium-high conversions
Table S2.
Parameters for calculating the reactivity ratio for the copolymerization of S (M
1
) and T(M
2
) from the extended Kelen-Tudos
( eKT
) model.
Run numb.
F f
ζ2 ζ1
Z G H η ξ
1
3.0000 1.6077 0.3177 0.1702 0.4882 1.2447 6.7452 0.1586 0.8594
2
1.5000 1.0719 0.2910 0.2079 0.6779 0.1060 2.3327 0.0308 0.6788
3
1.0000 0.9098 0.3351 0.3048 0.8911 -0.1012 1.1458 -0.0450 0.5094
4
0.6667 0.7235 0.2941 0.3192 1.1038 -0.2505 0.5938 -0.1476 0.3499
5
0.3333 0.4938 0.3067 0.4544 1.6538 -0.3061 0.1805 -0.2384 0.1406 a
Reaction conditions: [S+T]/[initiator]/[CuBr]/[PMDETA]=100:1:1:1, monomer/cyclohexanone=1/1
(g/mL), T=85°C
In the eKT method, the partial molar conversions for the two comonomers are defined as:
ζ2 = W (μ+ F ) / (μ+ f ) (S1)
ζ1 = ζ2* ( f/F ) (S2)
3
Figure S3.
A plot of η versus ζ from the data in Table S1. where W is the weight conversion of the polymerization, and μ is the ratio of molecular weights of
Monomer 2 compared to that of Monomer 1, with F and f are defined as before.
Then:
Z = log ( 1 -ζ 1 ) /log ( 1 - ζ2 ) (S3)
With the Z factor, η and ξ are equal to G /( α+ H ) and H /( α+ H ), respectively. Meanwhile G and
H are defined as ( Y
-
1)/ Z and Y / Z
2
, respectively.
According to the eKT method, the different parameters shown in Table S2 and ζ were plotted versus η as shown in Figure S3. The r
1
and r
2 values were calculated by Equation S4 from the slope and intercept, and were found to be r
1
= 0.22 and r
2
=
0.35, respectively.
( r
1
r
2
r
2
(S4)
2. At low conversions
Table S3.
Parameters for calculating the reactivity ratios determined for the copolymerization of S (M
1
) and T (M
2
) using the Finemann-Ross, Inverted Finemann-Ross, and the Kelen-Tudos models.
a
ζ= H /(α+ H ) Feed
Ratio
F =
( M
1
/ M
2
) b
Conv.
(%) c
Composition f =
( m
1
/ m
2
) d
G =
F -( F / f )
H =
F * F / f
η=
G
/(α+
H ) e
M
1
/ M
2 m
1 m
2
85/15 5.6667 8.63 0.6778 0.3222 2.1041 2.9735 15.2610 0.1745
75/25 3.0000 11.84 0.6063 0.3937 1.5401 1.0520 5.8439 0.1379
60/40 1.5000 11.41 0.5169 0.4831 1.0698 0.0979 2.1032 0.0252
50/50 1.0000 16.95 0.4783 0.5217 0.9170 -0.0906 1.0906 -0.0315
40/60 0.6667 12.10 0.4277 0.5723 0.7473 -0.2255 0.5947 -0.0948
25/75 0.3333 16.26 0.3581 0.6419 0.5580 -0.2641 0.1991 -0.1332
0.8953
0.7661
0.5411
0.3794
0.2500
0.1004 a
Reaction conditions: [S+T]/[initiator]/[CuBr]/[PMDETA]=100:1:1:1, monomer/cyclohexanone=1/1
4
(g/ml), T=85°C; b monomer feed composition (mole ratio); c determined via
1
H NMR analysis of sample collected directly from the reaction mixture without purification; d copolymer composition (mole ratio) as determined via
1
H-NMR; e α = 1.7431 (square root of the product of the least and the largest H values).
In the Finemann-Ross, G is plotted against H , as shown in Figure S4. The monomer reactivity ratios of S and T were determined to be r
1
=0.22 and r
2
=0.32 from the slope and intercept calculated by
Equation S5 of the best linear fit.
F f - 1
f
r
1
F
2 f
r
2
(S5)
Figure S4 . A plot of G versus H based on the data shown in in Table S3.
In the inverted Finemann-Ross method, G / H was plotted against 1/ H , as shown in Figure S5. The reactivity ratios of the monomers, r
1
=0.20 and r
2
=0.31, were calculated using Equation S6 from the slope and intercept of the inverted Finemann-Ross plot. f
F
- 1
r
2 f
F
2
r
1
(S6)
Figure S5.
The G / H versus 1/ H plot from the data in Table S3.
According to the Kelen–Tudos method, the notation of different variables have been explained in
5
Table S3. ζ was plotted versus η as shown in Figure S6. The r
1
and r
2 values calculated by Equation S7 from the slope and intercept, are 0.22, r
2
=0.32 respectively.
( r
1
r
2
r
2
(S7)
Figure S6.
The η versus ζ plot from the data in Table S3.
Composition drifts and microstructure variation during polymerization
The copolymer cumulative composition (CM) reflects the chemical composition of the copolymer after a certain period during the polymerization. The CM can be directly obtained via compositional analysis of the polymer from aliquot samples collected during polymerization using
1
H NMR technique to compare the intensity of the polymer signals at 6.7-7.5 ppm and 2.8–4.6 ppm. Meanwhile the instantaneous composition (IM) provides more information about how a copolymer’s chemical composition changes during the reaction. Instantaneous composition is calculated using Equation S8 as below:
F (M1) = ins i
< DP(M1) < DP(M1)
=
< DP(M1)+ < DP(M 2) < DP(total)
= conv F i cum
(M1) - conv i i-1
F cum
(M1) i-1
(S8) i-1 where F ins
(M1) i
represents the instantaneous composition of M1, conv i
is the total conversion of both monomers calculated by
1
H NMR, and F cum
(M1) is the cumulative composition of M1. A series of
ATRP reactions of S (M
1
) and T (M
2
) at different feed ratios were carried out. Cumulative composition and instantaneous composition of the S and T copolymers were evaluated.
6
Figure S7.
Dependence of the triad fraction of copolymer on the overall monomer conversion derived from
1
H NMR analysis ( S/T=1/1). The dashed lines represent the dependence of theoretical triad values on the overall monomer conversion.
Figure S8.
Dependence of the triad fraction of copolymer on the overall monomer conversion derived from
1
H-NMR analysis ( S/T=3/1). The dashed lines represent the dependence of the theoretical triad values on the overall monomer conversion.
7