decomposition carbonyl
advertisement

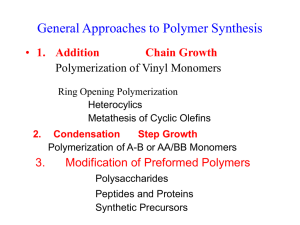
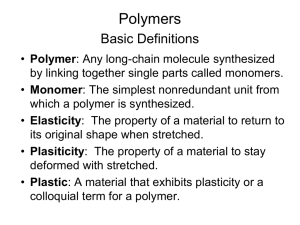
1. Polymerization 1.1 Classification of Polymers and Polymerization Mechanisms In 1929 W. H. Carothers suggested a classification of polymers into two groups, CONDENSATION and ADDITION polymers. Condensation polymers are those in which the molecular formula of the repeat unit of the polymer chain lacks certain atoms present in the monomer from which it is formed(or to which it can be degraded) for example, a polyester is formed by typical condensation reaction between bifunctional monomers, with elimination of water xHO R OH xHOCO R'COOH HO[ R OCO R'COO ]X H ( 2 X 1)H 2O The most important group of addition polymers includes those derived from unsaturated vinyl monomers CH 2 CH X CH 2 CH CH 2 CH , etc. X X Carother's original distinction between addition and condensation polymers was amended by Flory, who placed emphasis on the mechanism by which the two types of polymer are formed. Condensation polymers are usually formed by the stepwise intermolecular condensation of reactive groups; addition polymers ordinarily result from chain reaction involving some sort of active center. Some of the consequences of the differences between the mechanisms of chain and stepwise polymerization are shown in Table 2.1 Table 1.1 Distinguishing features of chain- and steppolymerization mechanisms Chain Polymerization vi) 1.1 Only growth reaction adds repeating units one at a time 1.2 to the chain. vii) 1.3 Monomer concentration decreases steadily throughout 1.4 reaction. viii)1.5 High polymer is formed at once; polymer molecular 1.6 weight changes little throughout reaction. 1.7 ix) Long reaction times give 1.8 high yields but affect molecular weight little. 1.9 x) Reaction mixture contains only monomer, high polymer, and about 10-8 part of growing chain. Step polymerization i) Any two molecular species present can react. ii) Monomer disappears early in reaction : at DP 10, less than 1% monomer remains. iii) Polymer molecular weight rises steadily throughout reaction. iv) Long reaction times are essential to obtain high molecular weights. v) At any stage all molecular species are present in a calculable distribution. 1.2 Step-Reaction(Condensation) Polymerization The type of product formed in a condensation reaction is determined by the functionality of the monomers, that is by the average number of reactive functional groups Per monomer molecule. Mono functional monomers give only low-molecularweight product. Bifunctional monomers give linear polymers Polyfunctional monomers, with more than two functional group per molecule, give branched(three dimensional) polymers. The properties of the linear and threedimensional polymers differ widely. The mechanisms of some step-reaction polymerization is discussed below. i) Carbonyl Addition-Elimination mechanism The most important reaction that has been used for the preparation of condensation polymers O R-C-X + Y : O: R-C-Y O R-C-Y + X: X Where R and R'( below) may be alkyl or aryl groups, X may be OH, .OR', NH2 NHR', OCOR' or Cl; Y may be R'OH, R'NH2 or R2COO-. i) Interchange The reaction between a glycol and an ester, XHO-R-OH + xR''OCO-R'-COOR'' R''O(-CO-R'-COO-R-O)xH + (2x-1)R''-OH Is often used to produce polyester, especially where the dibasic acid has low solubility. Frequently the methyl ester is used, as in the production of poly(ethylene terephthalate) from ethylene glycol and dimethyl terephthalete. The reaction between a carboxyl and an ester link is much slower, but other interchange reactions, such as amine-amide, amine-ester, and acetal-alcohol, are well known. iii) Carbonyl Addition – Substitution Reaction The reaction of aldehydes with alcohols, involving first addition and then substitution at the carbonyl groups, is of great practical and historic importance in stepwise polymerization. The general reaction, leading to acetal formation, is O RCH +R'OH OH RCH OR' OR' R'OH RCH OR' In addition to polyacetals, important polymers formed in this way include those of formaldehyde and phenol, urea or melamine iv) Nucleophilic Substitution Reaction These reactions are important from the standpoint of commercial organic polymers, primarily because of their lose in the polymerization of epoxides. The most common epoxide monomer is epichlorohydrin which reacts with a nucleophile N: as follows N: + H2C CHCH2Cl NCH2CHCH2Cl O: O Typically, the nucleophile is a bifunctional hydroxy compound such as bisphenol A and reaction proceeds as described below O __ CH2 CHCH2Cl + HO_ CH3 _ _ C _ OH CH3 v) Double-Band Addition Reaction Although addition reaction at double bands are often as associated with polymerization by chain mechanism this is not necessarily the case, and many important stepwise polymerization are based on this reaction. Of major interest among is the addition of dials to diisocyanates in the production of polyurethanes O xHOROH +xOCNR'CNO [ OROCNHR'NHC-]x 1.3 Radical Chain (Addition) polymerization The characteristics of chain polymerization listed in Table 2.1 of several postulated types of active center, three have been found experimentally: cation, anion and free radical. Free – radical polymerization is discussed in this chapter, and the related cases of ionic and coordination polymerization may be discussed later. The concept of vinyl polymerization as a chain mechanism is not new, dating back to Staudinger’s work in 1920. In 1937 Flory showed conclusively that radical polymerization proceeds by and requires the steps of Initiation Propagation and Termination Typical of chain reactions in low-molecular weight species. The carbon-carbon double band is, because of its relatively low stability, particularly susceptible to attach by a free radical. The reaction of the double band with a radical proceeds Well for compounds of the type CH2=CHX and CH2=CXY called vinyl monomers. Mot cell vinyl monomers yield high polymer as a result of radical aliphatic hydrocarbons of her then ethylene not at all. 1.3.1 Generation of Free Radicals Many organic reactions take place through intermediates having an add number of electrons and, consequently, an unpaired electron. Such intermediates are known as FREE RADICALS. They can be generated in a number of ways, including thermal decomposition of organic peroxides or hydroperoxides (Mageli 1968 or azo or diazo compounds (Zand 1965) Two reactions commonly used to produce radicals for polymerization are thermal or photochemical decomposition of benzoylperoxide 2C6H5COO- (C6H5COO)2 2C6H5. + 2CO2 And azobisisobutyronitrile (AIBN) (CH3)2CN CN NC(CH3)2 2(CH3)2C. + N2 CN Free radicals can be produced; by Thermal or photochemical decomposition of initiators such as Benzoylperoxide or AIBN High energy radiation; from a wide variety of sources including electrons, gamma rays, X-rays, and slow neutrons, is effective in producing free radicals that can initiate polymerization 1.3.2 Polymerization steps i) Initiation When free radicals are generated in the presence of vinyl monomer the radical adds to the double band with the regeneration of another radical If the radical formed by decomposition of the initiator I is designated R. I 2R. H 2R. + CH2 CHX RCH2C. X ii) Propagation The chain radical formed in the initiation step is capable of adding successive monomers to propagate the chain. H R (CH2CHX )XCH2C. + CH2 H H CHX R (CH2CHX )X+1CH2C . X iii) Termination Propagation would continue until the supply of monomer was exhausted were it not for the strong tendency of radicals to react in pairs to form a paired-electron covalent band with loss of radical activity. This tendency is compensated for in radical polymerization by the small concentration of radical species compared to monomers. The termination step can take place in two ways: Combination or coupling H H H CH2C. + .CH2___ ___ X ___ H CH2C____CH2 ____ X X X or disproportionation H H H . CH2C. + CH2___ ___ X CH2C___H H + C = CH___ X X X In which hydrogen transfer results in the formation of two molecules with one saturated and one unsaturated end group iv) configuration of monomer Units in Vinyl Polymer chains The addition of a vinyl monomer to a free radical can take place in either of two ways: R-CH2-CH. 2R + CH2= CHX (I) X R-CH-CH2. X (II) The reaction leading to the more stable product being favored. Since the unpaired electron can participate in resonance with the substituent X in structure I but not II, reaction I is favored. Steric factors also favor reaction I. The occurrence of reaction I exclusively would lead to head-to-tail configuration in which the substituents occur on alternate carbon atoms. _ _ _ _ _ _ _ _ CH2 CH CH2 CH CH2 CH CH2 X X X Alternative possibilities are a head-to-head, tail-to tail configuration _ _ _ _ _ _ _ _ CH2 CH CH CH2 CH2 CH CH X X X X Or random structure containing both arrangements. 1.4 Copolymerization Although the polymerization of organic compounds has been known for over 100 years, the simultaneous polymerization (Copolymerazation) of two or more monomers was not investigated until about 1911, when copolymers of olefins and diolefins were found to have rubbery properties and were useful than homopolymers made from single monomers. In the 1930’s it was found that monomers differed markedly in their tendencies to enter into copolymers. Staudinger (1939) fractionated a vinyl chloride-vinyl acetate copolymer made from a mixture of equimolar Quantities of the two monomers. He found no polymer containing equal amounts of each monomer but, instead, found vinyl chloride: vinyl acetate ratios of 9:3, 7:3, 5:3 and 5:7 among the fractions. In 1936 Dostal made the first attach on the mechanism of copolymerization by assuming that the rate of addition of monomer to a growing free radical depends only on the nature of the group on the chain. Thus monomers M1 and M2 lead to radicals of types M1. and M2. There are four possible ways in which monomer can add Reaction M1. + M1 M 1. rate k11[M1.][M1] M1. + M2 M 2. k12[M1.][M2] M2. + M1 M 1. k21[M2.][M1] M2. + M2 M 2. k22[M2.][M2] The monomer reactivity ratios r1 and r2 are the ratios of the rate constant for a given radical adding its own monomer to the rate constant for its adding the other monomer. r1>1 means that the radical M1. prefer to add M1 r1<1 means that it prefers to add M2 In the system styrene (M1)-methyl methacrylate (M2), for example, r1= 0.52 and r2 = 0.46 each radical adds the other monomer about twice as fast as its own. Ideal system :A copolymer system is said to be ideal the two radical show the same preference for adding one of the monomers over the other k11/k12 = k21/k22 or r1=1/r2 or r1r2=1 In this case the end group on the growing chain has no influence on the rate of addition, and the two types of units are arranged at random along the chain in relative amounts determined by the composition of the feed and the relative reactivities of the two monomers. 1.4.1 Polymerization Conditions and Polymer Reactions As the industrial production of polymers has increased over the years, there has developed an increasing interest in the application of chemical engineering principles to polymerization. Table 1.2 Comparison of polymerization System Type Bulk( batch type) Bulk(continuous) Solution Heterogeneous Suspension Emulsion Advantages Minimum contamination simple equipment for making casting Disadvantages Strongly exothermic Broadened molecular weight. Distribution at high conversion. Complex is small particles required Lower conversion per Requires agitation, pass leads to better material transfer, heat control and Separation and narrower molecular Recycling weight distribution Ready control of heat of Not use for dry polymer polymerization. Solution because of difficulty of may be directly useable. complete solvent removal Ready control of heat of Continuos agitation polymerization. required. Suspension of Contamination by resulting granular stabilized possible polymer may be washing, drying, directly useable possibly compacting required Rapid polymerization to high molecular weight and narrow distribution, with ready heat control. Emulsion may be directly useable.