File
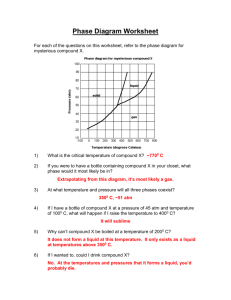
advertisement

Chapter 11 Practice ws #2 Objective # 7, 8, 9 Name: ______________________________ Date: ________ Hour: ______ 1. The solubility of oxygen in water at 0C and 1 atm is 0.073 g per liter. What is k in Henry’s law for this temperature? 2. The solubility of nitrogen at 0C in water is 8.21 X 10-4 mol/L if the N2 pressure above the water is 0.790 atm. What is the solubility at 1 atm of N2 and 0C? 3. What is the Henry’s law k for nitrogen in Problem 2? 4. The solubility of nitrogen in blood at 37C and 0.80 atm 5.6 X 10-4 M. If a deep sea diver breathes compressed air from a tankat a partial pressure of 3 atm, what would the solubility of nitrogen be in the diver’s blood at 37C? 5. The solubility of CO2 in water at 25C and 1 atm is 0.034 M. What is the Henry’s law constant (k)? What would the solubility of CO2 in water be at 0.038 atm and 25C? 6. The vapor pressure of ethanol at 40C is 135.3 torr. Calculate the vapor pressure of a solution of 53.6 g of glycerin, C3H8O3, in 133.7 g of ethanol, C2H5OH, at 40C. 7. Antifreeze solutions are mainly ethylene glycol, C2H6O2, in water. Calculate the vapor pressure of a solution made by adding 101.6 g of ethylene glycol to 139.6 g of water at 50.0C. At this temperature ethylene glycol is essentially nonvolatile, and the vapor pressure of water is 92.51 torr. 8. Sucrose, C12H22O11 (a nonvolatile substance), is a sweetener. A solution was made with 35.2 g of sucrose and 78.0 g of water at 30C. Calculate the vapor pressure of the solution if the vapor pressure of water at 30C is 31.824 torr. 9. How many grams of a nonvolatile compound B (molar mass = 97.8 g/mol) would need to be added to 250 g of water to produce a solution with a vapor pressure of 23.756 torr? The vapor pressure of water at this temperature is 42.362 torr. 10. Calculate the vapor pressure of a solution made with 321 g of toluene (C7H8) and 398 g of benzene (C6H6). At 60C, the vapor pressure of the two volatile components are 140. torr and 396 torr, respectively. 11. What is the vapor pressure of a solution made by adding 26.93 g potassium sodium tartrate, KNaC4H4O6 to 676.5 g of water at 25C? The vapor pressure of pure water is 23.756 torr at 25C. Assume that potassium sodium tartrate is nonelectrolyte (a poor assumption).