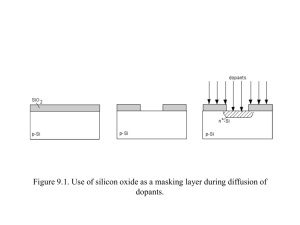
Section 8.2, Mastering Concepts Chapter 8, p.274 88. Explain how
advertisement

Section 8.2, Mastering Concepts Chapter 8, p.274 88. Explain how molecular compounds are named. Naming follows a specific set of rules. Those beginning with hydrogen are acids. Learn names of most used acids. For binary forms use names of nonmetals adding Greek prefixes to indicate number of atoms. Rember to change ending of last name to ide. 89. When is a molecular compound named as an acid? When it releases H+ in a water solution. Use acid names ifaqueous. Formulas usually start with hydrogen. 90. Explain the difference between sulfur hexafluoride and disulfur tetrafluoride. Sulfur hexafluoride is SF6, which has one atom ofsulfur bonded with six atoms of fluorine. Disulfur tetrafluoride is S2F4, which has two atoms ofsulfur bonded with four atoms of fluorine. 91. Watches The quartz crystals used in watchesare made of silicon dioxide. Explain how you use the name to determine the formula for silicon dioxide The name silicon indicates one atom of Si. Theprefix di- means two and oxide indicates oxygen.The correct formula is SiO2. 93. Name each molecule. a. NF3 b. NO c. SO3 d. SiF4 96. nitrogen trifluoride nitrogen monoxide sulfur trioxide silicon tetrafluoride Write the formula for each molecule. a. silicon dioxide b. bromous acid c. chlorine trifluoride d. hydrobromic acid SiO2 HBrO2 ClF3 HBr