Dating Rocks and Fossils Using Radioactive Isotopes
advertisement
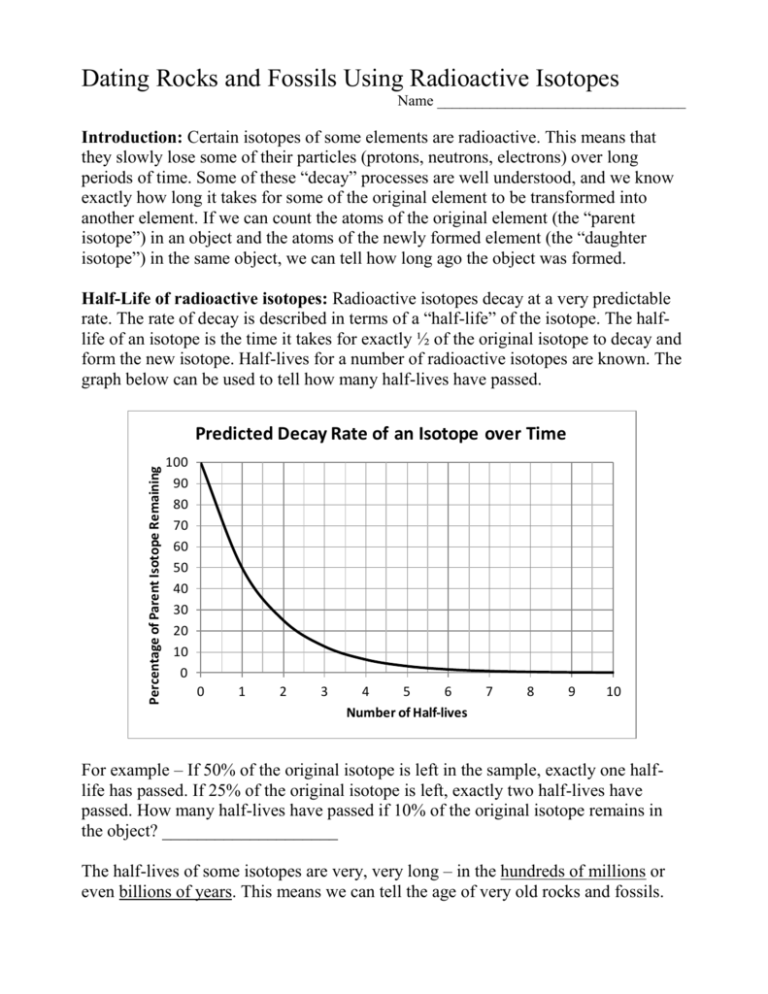
Dating Rocks and Fossils Using Radioactive Isotopes Name _________________________________ Introduction: Certain isotopes of some elements are radioactive. This means that they slowly lose some of their particles (protons, neutrons, electrons) over long periods of time. Some of these “decay” processes are well understood, and we know exactly how long it takes for some of the original element to be transformed into another element. If we can count the atoms of the original element (the “parent isotope”) in an object and the atoms of the newly formed element (the “daughter isotope”) in the same object, we can tell how long ago the object was formed. Half-Life of radioactive isotopes: Radioactive isotopes decay at a very predictable rate. The rate of decay is described in terms of a “half-life” of the isotope. The halflife of an isotope is the time it takes for exactly ½ of the original isotope to decay and form the new isotope. Half-lives for a number of radioactive isotopes are known. The graph below can be used to tell how many half-lives have passed. Percentage of Parent Isotope Remaining Predicted Decay Rate of an Isotope over Time 100 90 80 70 60 50 40 30 20 10 0 0 1 2 3 4 5 6 Number of Half-lives 7 8 9 10 For example – If 50% of the original isotope is left in the sample, exactly one halflife has passed. If 25% of the original isotope is left, exactly two half-lives have passed. How many half-lives have passed if 10% of the original isotope remains in the object? ____________________ The half-lives of some isotopes are very, very long – in the hundreds of millions or even billions of years. This means we can tell the age of very old rocks and fossils. Procedure: Choose one of the 5 bags of “atoms” (A,B,C,D,or E). There is a card with each bag explaining what isotopes are represented by the “atoms” in the bag. In each bag, there are three types of objects: the parent isotope, the daughter isotope, and objects representing all of the other atoms in the sample (you do not need to do anything with these extra atoms). 1. Count the number of parent isotopes in the bag. Enter this number in the data table. 2. Count the number of daughter isotopes in the bag. Enter this number in the data table. [NOTE: PLEASE DO NOT LOSE ANY OF THE OBJECTS.] 3. Calculate the % of the parent isotope remaining in the object: % parent isotope remaining = (# of parent isotopes) (# of parent isotopes + # of daughter isotopes) X 100 Enter the % in the data table. 4. Use the graph on page 1 to determine how many half-lives have passed since the object was formed. Be very exact when reading the graph – estimate carefully. Enter this number in the data table. 5. Multiply the # of half-lives by the half-life of the isotope in your bag (printed on the card with the bag). This is the age of your sample in years. Enter the age in the data table. 6. Put ALL of the “atoms” back into the bag and seal the bag. Choose another bag and repeat the procedure. Do all 5 bags if possible. Data Table Rock Isotope Half- # of # of % of Number or used life parent daughter parent of halfFossil isotopes isotopes isotope lives Letter A B C D E Isotopes used: Uranium 235; half-life of 704 million years. Uranium 238; half-life of 4.5 billion years. Thorium 232; half-life of 14 billion years. Age in years Questions: 1. Which sample was the oldest? ______ How old was it? ____________________ Knowing what you know about the early earth, do you think any real rocks or fossils could be this old? ____________ Explain your answer. ________________________ ____________________________________________________________________ 2. If you have a YOUNG sample and a very OLD sample of a certain rock type, which has more of the parent isotope? _________ Explain why, in your own words. ____________________________________________________________________ ____________________________________________________________________ 3. If you have a fossil that you think is about 200 million years old, which isotope would you use to date it – Thorium 232, Uranium 235, or Uranium 238? __________ Explain why, in terms of the graph we used. ________________________________ ____________________________________________________________________ 4. Suppose a geologist dated a rock using U-235, and only the daughter isotope Pb207 was found (no atoms of U-235 were detected). What could be said about the age of the rock? __________________________________________________________ [Think – use the graph on page 1 in thinking about this question.] If you really needed to know a more exact age for the rock, what could you do? ____________________________________________________________________ 5. PLEASE READ: Carbon 14 (C-14) is an isotope of carbon used to date more recent objects that were once living, like bones and hair of early Humans, fabric, charcoal, wood, leather, seeds, pollen, etc. For example, the “Dead Sea Scrolls” and the mummies in Egyptian pyramids were dated using C-14. Carbon 14 decays to nitrogen 14, with a half-life of 5730 years. a. Use the graph to see how much C-14 would be left in a sample after 2 half-lives (11,460 years). (What % of the original C-14 is left?) _________________________ b. How about after 3 half-lives (17,190 years)? _________________ c. ABOUT how many half-lives could pass before the C-14 in the sample would be such a small amount that it couldn’t be detected? _____________ Calculate how many years this would be (multiply # of half-lives x 5730 years). ________________ (This is about the maximum age that can be dated using C-14.) d. Could C-14 be used to date any of the “samples” we used in this activity? _______ Why not? ____________________________________________________________









