Ionic and Covalent Bonds Activity
advertisement
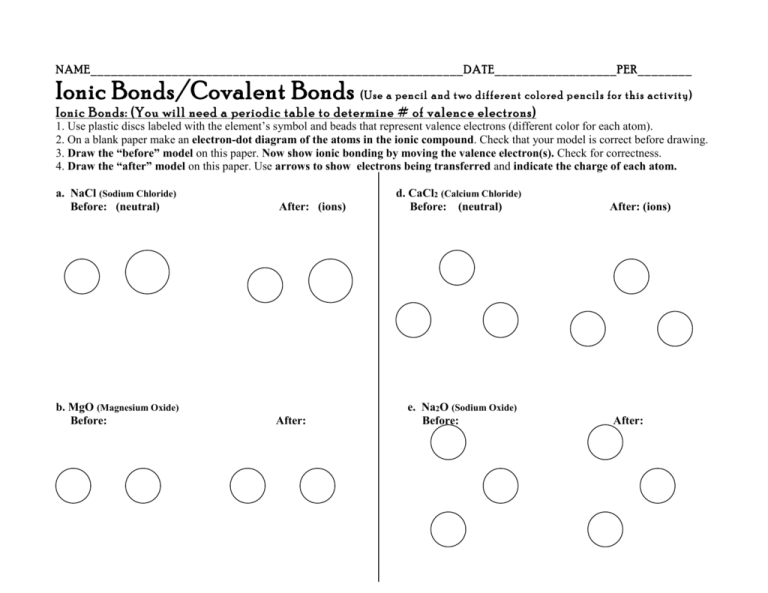
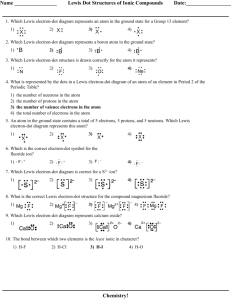
NAME_______________________________________________________DATE__________________PER________ Ionic Bonds/Covalent Bonds (Use a pencil and two different colored p encils for this activity) Ionic Bonds: (You will need a periodic table to determine # of valenc e electrons) 1. Use plastic discs labeled with the element’s symbol and beads that represent valence electrons (different color for each atom). 2. On a blank paper make an electron-dot diagram of the atoms in the ionic compound. Check that your model is correct before drawing. 3. Draw the “before” model on this paper. Now show ionic bonding by moving the valence electron(s). Check for correctness. 4. Draw the “after” model on this paper. Use arrows to show electrons being transferred and indicate the charge of each atom. a. NaCl (Sodium Chloride) Before: (neutral) After: (ions) b. MgO (Magnesium Oxide) Before: After: d. CaCl2 (Calcium Chloride) Before: (neutral) e. Na2O (Sodium Oxide) Before: After: (ions) After: Covalent Bonds 1. Make a model with atom discs and electron beads. Show how electrons are shared in a covalent bond. Check that it is correct. 2. Next draw it on this paper as as an electron-dot model . Use different colors for the valence electrons of each atom. 4. . Finally draw a model that uses a line to represent each pair of electrons that are shared. You may have double or single bonds. a. H2 (Hydrogen Molecule) Electron-dot Line to show each electron pair d. H2O (water) Electron-dot Line to show each electron pair b. O2 (oxygen molecule) Electron-dot Line to show each electron pair e. NH3 (ammonia) Electron-dot Line to show each electron pair c. CO2 (Carbon Dioxide) Electron-dot Line to show each electron pair f. CH4 (methane) Electron-dot Line to show each electron pair