Chemical and Physical Properties Review WS
advertisement

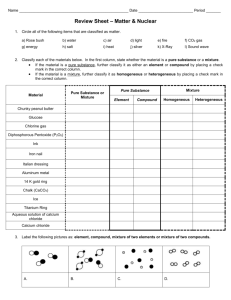
Name _____________________________________ Date ________________________ Period _______ Physical and Chemical Properties REVIEW 1. What is a PHYSICAL property? How can these be identified? 2. What is a CHEMICAL property? How can these be identified? 3. Define density. What is the equation for density? Is density a physical or a chemical property? Calculate the following: a. A solid has a mass of 80.0 grams and occupies 25.3 mL of space. What is its density? b. Liquid A has a volume of 32 mL and a mass of 13.3 grams, while Liquid B has a volume of 45 mL and a mass of 23.5 grams. What will these liquids look like when placed into a beaker? c. A liquid has a mass of 21.0 grams and a density of 6.7 g/mL. What is its volume? d. Another liquid has a density of 13.6 grams and a volume of 11.5 mL. What is its mass? 4. Create a tree diagram given the following: Air Calcium Chicken soup Compound Gold Heterogeneous mixture Matter Pure substance Sugar NaCl Tea Homogenous mixture 5. DEFINE: Element: Compound: Pure Substance: Mixture: Homogenous mixture: Heterogeneous mixture: Element Soil Mixture 6. Draw three particle diagrams to represent what happened as you melted the ice in class. Use the same number of particles in each. You are allowed to draw outside of the boxes. 7. Create a graph that shows what would happen if you started with boiling water and ended up with ice. Make sure to label and explain what is happening at each part of the graph. You should use complete sentences.