Draft Guidelines for Surgical Procedure Antibiotic
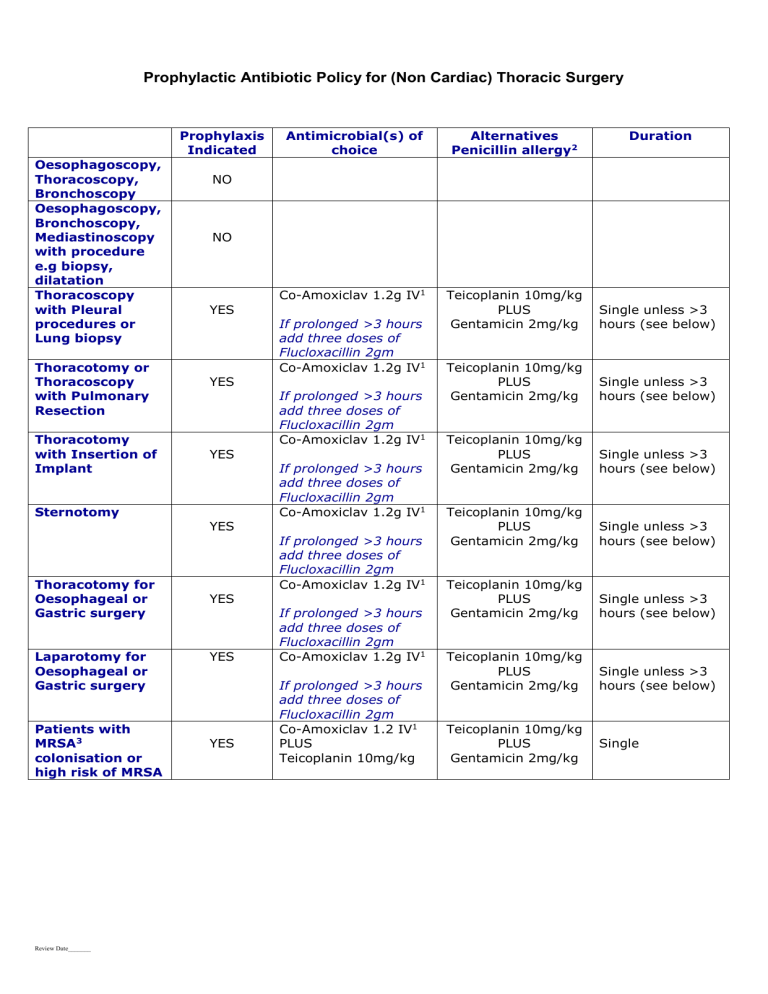
Prophylactic Antibiotic Policy for (Non Cardiac) Thoracic Surgery
Oesophagoscopy,
Thoracoscopy,
Bronchoscopy
Oesophagoscopy,
Bronchoscopy,
Mediastinoscopy with procedure e.g biopsy, dilatation
Thoracoscopy with Pleural procedures or
Lung biopsy
Prophylaxis
Indicated
NO
NO
YES
Thoracotomy or
Thoracoscopy with Pulmonary
Resection
Thoracotomy with Insertion of
Implant
Sternotomy
Thoracotomy for
Oesophageal or
Gastric surgery
Laparotomy for
Oesophageal or
Gastric surgery
Patients with
MRSA 3 colonisation or high risk of MRSA
YES
YES
YES
YES
YES
YES
Antimicrobial(s) of choice
Co-Amoxiclav 1.2g IV 1
If prolonged >3 hours add three doses of
Flucloxacillin 2gm
Co-Amoxiclav 1.2g IV 1
If prolonged >3 hours add three doses of
Flucloxacillin 2gm
Co-Amoxiclav 1.2g IV 1
If prolonged >3 hours add three doses of
Flucloxacillin 2gm
Co-Amoxiclav 1.2g IV 1
If prolonged >3 hours add three doses of
Flucloxacillin 2gm
Co-Amoxiclav 1.2g IV 1
If prolonged >3 hours add three doses of
Flucloxacillin 2gm
Co-Amoxiclav 1.2g IV 1
If prolonged >3 hours add three doses of
Flucloxacillin 2gm
Co-Amoxiclav 1.2 IV 1
PLUS
Teicoplanin 10mg/kg
Alternatives
Penicillin allergy 2
Teicoplanin 10mg/kg
PLUS
Gentamicin 2mg/kg
Teicoplanin 10mg/kg
PLUS
Gentamicin 2mg/kg
Teicoplanin 10mg/kg
PLUS
Gentamicin 2mg/kg
Teicoplanin 10mg/kg
PLUS
Gentamicin 2mg/kg
Teicoplanin 10mg/kg
PLUS
Gentamicin 2mg/kg
Teicoplanin 10mg/kg
PLUS
Gentamicin 2mg/kg
Teicoplanin 10mg/kg
PLUS
Gentamicin 2mg/kg
Duration
Single unless >3 hours (see below)
Single unless >3 hours (see below)
Single unless >3 hours (see below)
Single unless >3 hours (see below)
Single unless >3 hours (see below)
Single unless >3 hours (see below)
Single
Review Date_______
NOTES:
1 Single dose prophylaxis
The central proposal of this guideline is to adopt the principles of the SIGN guidance limiting antibiotic prophylaxis to a single dose in most instances. This is seen by the Medical Director, the Infection
Control and Prevention Team, the Microbiologists and the Infectious Disease Physicians as a central measure to control the incidence of C. Difficile , MRSA, ESBL and other multi-resistant pathogens.
The relevant exceptions to single dose prophylaxis would be:
1. When duration of surgery is >3 hours [this is particularly the case in oesophagectomies]
Co-Amoxiclav has shorter half life than Teicoplanin.
Three doses of Flucloxacillin 2gm should be given six hours apart commencing at
3 hours from the start of the operation [only when Co-Amoxiclav is used as single agent for prophylaxis].
2. When blood loss exceeds 1.5 litres (extra dose administered after fluid replaced)
2 Penicillin allergy
Beta lactams (such as penicillins and cephalosporins) are often the cornerstone of antibiotic prophylaxis. If a patient has been wrongly attributed with a penicillin allergy, optimal management may be compromised. Patient history is integral to evaluation of allergy. Therefore patients with a history of penicillin allergy should be reviewed to exclude a non-immunological adverse reaction, (for example, diarrhea, vomiting, non-specific maculopapular rash) or an experience wrongly attributed to the antibiotic (for example ampicillin and Epstein-Barr infection).
While a precautionary approach to penicillin or other drug allergy should be adopted, where a reliable history can be obtained the following definition of significant penicillin allergy should be used:
A history of a hypersensitivity reaction such as anaphylaxis, laryngeal oedema, bronchospasm, angioedema, hypotension, local swelling , urticaria, or pruritic rash, which occurs immediately following penicillin administration – or other life threatening reactions (e.g Stevens Johnson syndrome) –contraindicates further exposure to penicillin and beta lactams apart from aztreonam.
Late manifestations are only a relative contraindication.
3 MRSA
Patients who require surgery and have a history of MRSA colonisation or infection should be given
MRSA-colonisation eradication therapy, which is usually successful short term. They should also then receive pre-operative glycopeptide prophylaxis in combination with the other above antibiotic(s) active against other potential pathogens. This is because there may be an appreciable risk that patients’ MRSA carriage may have recurred before surgery. The use of glycopeptides may also be considered if patients come from facilities with a high prevalence of MRSA.
There are two glycopeptides available in the UK, Vancomycin and Teicoplanin. Although Vancomycin has been recommended in the Trust’s general antibiotic treatment policy, its administration involves a prolonged administration time of 100- 120 minutes. Given the importance of proper timing of antibiotic administration in Surgical Prophylaxis, and also for logistical and possibly nephroptoxicity reasons, Teicoplanin would be recommended in preference to Vancomycin.
Review Date_______
To summarise when MRSA cover is required add a single dose of Teicoplanin 10mg/kg IV to one of the above recommended antibiotic prophylactic regimens or alternatively choose a second line regimen already containing Teicoplanin if available.
Recommendation for patients with heart valve prosthesis and other prosthesis:
Antibiotic prophylaxis solely to prevent Infective Endocarditis (IE) is no longer recommended before surgical procedures. For patients with established infections in which enterococci may be part of the infecting bacterial flora and with one of the below-listed cardiac conditions associated with the highest risk of an adverse outcome from endocarditis, amoxicillin, or ampicillin should be included in the antibiotic treatment regimen for enterococcal coverage. Teicoplanin or Vancomycin may be substituted for patients allergic to or unable to tolerate amoxicillin or ampicillin.
Cardiac conditions associated with the highest risk of an adverse outcome from IE, include the following: (1) a prosthetic cardiac valve, (2) a history of previous IE, (3) cardiac transplant recipients who develop cardiac valvulopathy, and (4) patients with congenital heart disease (CHD), including (a) those with unrepaired cyanotic CHD (including palliative shunts and conduits), (b) those with completely repaired CHD with prosthetic material or device, placed surgically or by catheter, for the first 6 months
after the procedure, and (c) those with repaired CHD with residual defects at the site or adjacent to the site of a prosthetic patch or device .
STATEMENT OF INTENT
This guideline is not intended to be construed or to serve as a standard of care. Standards of care are determined on the basis of all clinical data available for an individual case and are subject to change as scientific knowledge and technology advance and patterns of care evolve. Adherence to guideline recommendations will not ensure a successful outcome in every case, nor should they be construed as including all proper methods of care or excluding other acceptable methods of care aimed at the same results.
The ultimate judgement must be made by the appropriate healthcare professionals responsible for clinical decisions regarding a particular clinical procedure or treatment plan. The judgement should only be arrived at following discussion of the options with the patient covering the diagnostic and treatment choices available. It is advised however that significant d epartures from the guideline should be fully documented in the patient’s case notes at the time the relevant decision is taken. The aim of this guideline is to identify the operations for which routine prophylaxis is supported by evidence. However the ul timate decision rests with the surgeon’s assessment of risk and benefit. Giving prophylaxis to patients who are having procedures for which this guideline does not recommend prophylaxis can be justified if the surgeon believes the patient to be at particularly high risk from Surgical Site Infection. In this case the criteria used for risk assessment should be recorded.
Review Date_______






