Lab Analysis of a Chemical Reaction July 20th 2011

ANALYSIS OF A CHEMICAL REACTION
Introduction:
During this lab you will be determining the correct chemical equation from these four possibilities:
A]
B]
C]
NaHCO
3
(s) → NaOH (s) + CO
2
(g)
2 NaHCO
3
(s) → Na
2
CO
3
(s) + CO
2
(g) + H
2
O (g)
2 NaHCO
3
(s) → Na
2
O (s) + 2CO
2
(g) + H
2
O (g)
4 NaHCO
3
(s) → 2Na
2
C
2
O
4
+ O
2
(g) + 2H
2
O (g) D]
Purpose:
To determine the mole ratio for a reactant and a product in a chemical reaction.
To use the mole ratio information along with qualitative evidence to identify the reaction from among four possibilities
Pre-Lab: /2 K/U /8 I
1) What is the formula of sodium bicarbonate? (1 K/U)
/5 C
Sodium bicarbonate or sodium hydrogen carbonate is the chemical compound with the formula NaHCO
3
= Baking Soda
2) What is the test for the presence of carbon dioxide? Why does it work? (1 K/U, 1 I)
Baking soda consists of the chemical compound sodium bicarbonate (NaHCO3).
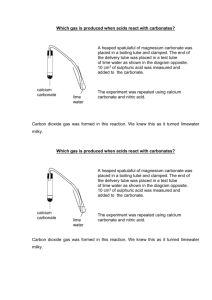
Compounds containing carbonate (CO3) react with acids such as vinegar (acetic acid) to produce carbon dioxide gas (CO2). The equation for this reaction is as follows:
Limewater, Ca (OH)
2
, is used to test for the presence of carbon dioxide gas because it reacts with carbon dioxide to form the compound calcium carbonate
(CaCO
3
). The equation for this reaction is as follows:
Note: The (s) in the equation indicates that calcium carbonate is insoluble. That is, it does not dissolve. The small white particles of insoluble calcium carbonate temporarily stay suspended in the solution and give it a milky appearance. In time, gravity pulls the calcium carbonate to the bottom of the jar.
*The Limewater Test is used to test for the presence of carbon dioxide.
3) How might you be able to demonstrate that water is being produced? (1 I)
When you heat the baking soda you will get water vapour(g) clinging to the walls of the test tube.
4) You will be deciding which reaction is correct based on both qualitative and quantitative observations. a) Explain how the qualitative observations will help you determine which reaction(s) are potentially correct for this experiment. (2 I)
The qualitative observations for this test would be that you would observe a white milky solution during the lime test.
During the heating process, water vapor and gas are produced; therefore,
H
2
O and a gas must be in the equation. b) How will quantitative observations be used to determine which reaction is correct? (2 I)
By calculating the amount of product that will be formed we can determine the quantitative observations.
The weighing of the sodium bicarbonate before and after heating will determine which reaction is correct.
5) Does the mass in step 1 of the procedure need to be exact, why or why not? (2 I)
When sodium hydrogencarbonate (baking soda) is heated, it decomposes to make sodium oxide, carbon dioxide and water. Therefore, the exact amount of baking soda is not required because the reaction will still occur and the product will still be formed, what will change is the amount of product that will be formed.
The mass of the sodium bicarbonate in step 1 does not have to be exact as it is merely a test to detect the presence of the byproduct of decomposition. We are not determining the weights of the byproducts at this time.
6) Draw a flow chart for this experiment.
(5 C)
#1
NaHC0
3
(s)
0.5g heat for 2 min.
Limewater for 2 min. shake
Materials:
test tubes
test tube clamp
Bunsen burner
Retort stand
Clay triangle
Crucible and cover
Heating pad (to protect lab bench)
ring clamp
Test tube tongs
Crucible tongs
Sodium bicarbonate
Lime water
Scale
NaHC0
3
(s)
0.2g heat for 2 min.
+ 8 min
Strong heat
Procedure:
1) Add about 0.5 g of sodium hydrogen carbonate to a test tube. Clamp this test tube on a right angle to the retort stand so that the sodium hydrogen carbonate is spread horizontally along the side of the tube. Heat the sodium hydrogen carbonate GENTLY for 2 minutes. Be sure not to heat the rubber on the test tube clamp – it will burn. Hold another test tube half-filled with limewater under the mouth of the test tube that is being heated (use test tube tongs). After the 2 minutes of heating shake the test tube containing limewater. What do you observe? – limewater turns milky. What do you notice on the sides of the test tube that contains sodium hydrogen carbonate? – water droplets
2)
Record both observations:
3) The observations from step one will help you narrow your choice of possible equations for the reaction. To determine which of the remaining possible reactions is the correct one you must obtain quantitative data. You will be completing the table below: (2 marks)
Mass of crucible (g)
Mass of crucible cover (g)
Mass of sodium bicarbonate (g)
Mass of crucible, cover and sodium hydrogen carbonate (g)
Mass of crucible, cover and product (after heating) (g)
Mass of product (g)
Ratio of Mass of sodium hydrogen carbonate: Mass of product
4) Measure the mass of a clean, dry crucible and cover. Add about 2.0 g of sodium hydrogen carbonate. The mass does not need to be exactly 2.0 g but should be measured as accurately as your measuring device allows. Determine and record the exact mass of the crucible, cover and sodium hydrogen carbonate.
5) Place the crucible, with its cover slightly agar, on a clay triangle supported by an iron ring on a ring stand. Heat gently for 2 minutes and then heat more strongly for 8 minutes. Place the crucible and cover on a heating pad and allow them to cool. Determine the mass of the crucible, cover and product.
Analysis
1.
What do you observe in the test tube that was heated in step # 1 (the one that contains the sodium hydrogen carbonate)? What does this indicate?
– white residue and water droplets on wall of test tube (1 A) ? – decomposition of sodium bicarbonate
2.
After you shook the test tube with limewater, what did you observe?
– limewater turned milky white. What does this indicate?(1 A) - presence of a gas (CO2) as it reacts with the lime to form scale
After the test tube with limewater was shaken it turned milky white which indicated that it contained carbon dioxide gas. Since you cannot see carbon dioxide, this is a great test to use to indicate the presence of carbon dioxide gas.
3.
On the basis of the observations after step #1, which of the four choices can be ruled out?
Why? (1 A)
D] 4 NaHCO
3
(s) → 2Na
2
C
2
O
4
+ O
2
(g) + 2H
2
O (g)
Because the equation does not produce carbon dioxide we can thus rule out D.
But also Choice A] can be ruled out as it does not contain any water in the equation. And you would observe water droplets on the side of the test tube. (1 A)
4.
Theoretically, what is the value for the ratio of the mass of sodium hydrogen carbonate: mass of solid product, for each of the possible reactions not ruled out by qualitative observations? Remember that the masses of the gases will not be observed in your experiment and so you are only calculating the ratio of the solid masses in the equations.
Clearly show all of your calculations. (3 A)
Molar
Ratio mass
2 NaHCO
3
(s)
→
Na
2
CO
3(s)
+
2 1
Molar mass
0.5 g
Na: 23 X1 = 23
H: 1 X 1 = 1
C: 12 X1 = 12
O: 16 x3 = 48
84g/mol moles mass (g) / molar mass g/mol
0.5g/84 g/mol =
0.00595238mol
=5.952X10-3 mol
2:1 therefore mole = ½
0.00297619mole
Na: 23 X2 = 46
C: 12 X1 = 12
O: 16 x3 = 48
106g/mol
Mass(g)=
2.97619 X10-3
X106g/mol
= 0.3154g
CO
1
2
(g) + H
1
2
O (g)
5.
What is the experimentally determined mass ratio? Which of these ratios agrees with the experimentally determined ratio? Write the correct chemical equation of the decomposition of sodium hydrogen carbonate.(2 A)
B] 2 NaHCO
3
(s) → Na
2
CO
3
(s) + CO
2
(g) + H
2
O (g)
6.
Now that you know the identity of the solid product, calculate the experimental ratio of moles of sodium hydrogen carbonate to moles of solid product. Clearly show all of your calculations. (2 A
See above- the experimental ratio of moles of sodium hydrogen carbonate to moles of solid product.
Sodium hydrogen carbonate : solid product Na
2
CO
3
(s
0.00595238mol : 0.00297619mole
7.
How does your answer to #6 compare to the mole ratio of the balanced chemical equation you have chosen? (1 A)
2: 1 ratio
Marking Scheme for Lab:
10 marks Communication based on communication rubric and proper lab format
20 marks Application (2 data table, 11 analysis, 2 error analysis , 2 conclusion, 3 lab performance)
/20 A /10 C