The Family Practice Newsletter - Doctors Hospital Family Practice
advertisement

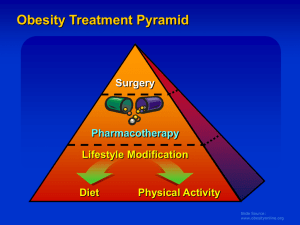
The Family Practice Newsletter The Ohio University College of Osteopathic Medicine The Ohio Northern University Raabe College of Pharmacy Doctors Hospital Family Practice Volume 6, Issue 7 February, 2007 Recent Gains in Weight Loss Scott Amstutz, ONU Doctor of Pharmacy Candidate Obesity is defined as increased body weight caused by excessive accumulation of fat. Over 60% of Americans are classified as overweight (BMI between 25 and 29.9 kg/m2) or obese (BMI > 30 kg/m2). Those with excess weight place themselves at an increased risk for many health problems including diabetes, cardiovascular diseases, sleep apnea, and some types of cancer. The recommended starting point for weight loss is diet and exercise. A healthy diet should contain 30% or less of the total daily calories as fat, and include many fruits and vegetables. The recently popular “fad” diets should be avoided as they can teach improper eating habits and are only temporarily effective. The focus should be on calorie control: making sure that the number of calories consumed each day is less than the calories used during daily activities and exercise. Patients who want to lose weight need to incorporate regular exercise into their daily routines as it can provide additional benefits over just weight loss. It is recommended to get at least 30 minutes of moderate intensity exercise most days of the week. When diet and exercise are not enough, drug therapy may be necessary. Use of drug therapy may be considered for those patients who are mild to moderately overweight and have medical complications that would improve from moderate weight loss. Drug therapy should be used as an adjunct to the above mentioned lifestyle modifications. Two FDA-approved drug classes for weight loss include appetite suppressants and digestion inhibitors. Appetite suppressants are central nervous system stimulants that decrease appetite by causing a feeling of fullness. Some drugs in this class include diethylpropion (Tenuate), and phentermine (Adipex-P). Dextroamphetamine (Dexedrine), also a CNS stimulant, is approved only as adjunct treatment for obesity. These CNS stimulants inhibit the reuptake of serotonin and norepinephrine. These stimulants are all considered controlled substances by the DEA, and are therefore monitored more closely than other medications. Common side effects include headache, dry mouth, constipation, insomnia, and the most concerning is an increase in blood pressure. Another anti-obesity agent, sibutramine (Meridia) works on many receptors in the body, effectively blocking the reuptake of serotonin, norepinephrine, and dopamine. Of these medications, only sibutramine is approved for the longterm management of obesity. Due to sibutramine’s effect on serotonin, a 14 day washout period is required with the use of MAO Inhibitors. Monitoring for all weight loss medications should include weight, waist circumference, blood pressure, heart rate, and any heart abnormalities. The only FDA-approved digestion inhibitor is orlistat (Xenical). It is a reversible lipase inhibitor in the lumen of the stomach and small intestine, which blocks the breakdown and absorption of dietary fats resulting in a reduced caloric intake. Orlistat has also been shown to decrease LDL cholesterol, fasting blood glucose, and insulin levels. The dose of orlistat is 120mg three times a day. Adverse events which can limit compliance include oily spotting, flatus with discharge, fecal urgency, and fatty stool. These may be decreased if the patient decreases the amount of fat in the diet. Since orlistat decreases the absorption of fat- soluble vitamins, it is recommended that patients take a multivitamin every day. An over-the-counter version of orlistat known as Alli (pronounced ALeye) was recently made available. It is half the dose (60 mg) of the prescription orlistat and has the same prescribing information. A new drug class may soon be entering the U.S. market, as it has already been approved in the European Union. Rimonabant (Acomplia, Zimulti in the U.S.) is a new anti-obesity drug that selectively inhibits the cannabanoid type 1 receptor. This receptor is one of two receptors found in the endocannabinoid system. This system is overactivated in obesity and results in excessive food intake and fat accumulation. Rimonabant therapy can lead to a reduction in insulin resistance, improved glucose tolerance, a reduction in triglycerides, and an increase in HDL cholesterol. The most common adverse effect reported with rimonabant was depression, due to inhibition of the cannabanoid receptor. Rimonabant is currently being evaluated for the as an aid to smoking cessation and for its positive effects on atherosclerosis. The FDA has currently delayed approval of rimonabant and will continue to analyze data until the end of July. With all of the patients concerned about their weight and wanting to lose it, many medications can contribute to gain weight. Medication-induced weight gain can be detrimental for many patients contributing to a decrease in compliance with their medications and worsening of disease states. Weight gain is most commonly associated with the following medications: antidepressants, antipsychotics, anticonvulsants/mood stabilizers, and hypoglycemic mediations. Antidepressants can cause weight gain when used for long-term therapy, especially the selective serotonin reuptake inhibitors: paroxetine (Paxil), sertraline (Zoloft), and fluoxetine (Prozac). Of these, paroxetine has been associated with the highest incidence of weight gain, with as much as 14 kg can be seen over 26 to 32 weeks. Other antidepressants showing weight gain include amitriptyline (Elavil), imipramine (Tofranil), and phenelzine (Nardil). Some alternative antidepressants that are not associated with weight gain, include bupropion (Wellbutrin), venlafaxine (Effexor), and desipramine (Norpramine). Patients on buproprion may actually experience weight loss, which is usually beneficial! Commonly used antipsychotic drugs have also been shown to cause weight gain, including chlorpromazine (Thorazine), clozapine (Clozaril), and olanzapine (Zyprexa). The weight gain seen with these is not dose related. Other agents showing similar benefits without weight gain include molindone (Moban), aripiprazole (Abilify), or ziprasidone (Geodon). Anticonvulsants/mood stabilizers have been shown to cause weight gain when used for long-term therapy. Lithium (Lithobid), pregabalin (Lyrica), and carbamazepine (Tegretol) are included in this group, with valproic acid (Depakote) associated with the largest, anywhere from 5 to 49 kg of weight gain with long-term treatment. If weight gain becomes problematic, consider switching to topiramate (Topamax), which may decrease weight, or zonisamide (Zonegran). Another common drug category causing weight gain, which can worsen diabetes, is the hypoglycemic medications. Insulin use is associated with increased weight, as weight gain can be directly proportional to the number of injections used per day. Using Lantus once daily may help to prevent the weight gain, and Byetta may cause weight loss in some cases. Other hypoglycemic classes commonly causing weight gain include the sulfonylureas: glipizide, glyburide, glimipiride; meglitinides: repaglinide; and TZDs: pioglitazone (Actos) and rosiglitazone (Avandia). The increased weight gain with the TZDs usually presents as lower extremity edema, which may exacerbate a patient’s CHF. Alternatives to these classes include metformin (Glucophage), and exenatide (Byetta). With treating diabetes with any of these medications, it should be remembered that adequate glucose control is more important than weight gain in most patients. Other medications may cause weight gain and results will vary from patient to patient. It is important to keep close communications between patients and doctors regarding medicine expectations and side effects, desired clinical results, and possible alternatives in drug therapy. Proper diet and exercise may help to prevent much of the weight gain seen with many of these medications. References: 1. 2. 3. Collazo-Clavell ML. Save and effective management of the obese patient. Mayo Clin Proc 1999; 74: 1255-1260 Fabricatore AN, Wadden TA. Treatment of obesity: an overview. Clin Diabetes 2003; 21: 67-72 FDA website. News concerning the approval of OTC orlistat 2007. Accessed 03/13/2007 http://www.fda.gov/bbs/topics/NEWS/2007/NEW01557.html 4. Wan-Chih T. Acomplia (rimonabant) and OTC orlistat for weight loss. Pharmacist’s Letter/Prescriber’s Letter 2006; 22: 220313