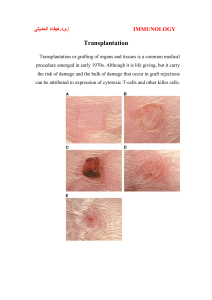
Transplantation immunology The transplantation of organs from
advertisement

Transplantation immunology The transplantation of organs from unrelated donors into recipient to replace damaged or diseased organs is now widely established. Cadaveric renal transplantation has become a routine treatment for end stage renal failure and transplantation of the heart, liver and pancreas, are being carried out with increasing frequency. Unless suitable and reliable artificial support systems become available or great advances in the prevention or early treatment of the original disease occur, neither of which seem likely in the near future, then the practice of organ transplantation will continue to increase. The major barrier to effective organ transplantation is rejection of the transplant by the recipient's immune system. Before discussing the immunological aspects in detail it is important to outline the terminology involved. Autograft: A graft taken from one part of an individual and transplanted to another site in the same individual, e.g. a skin graft in a burned patient. Isograft: a graft between genetically identical individuals e.g. renal transplantation between identical twins. Allograft: A graft between genetically non-identical individuals of the same species (syn. Homograft), e.g. a cadaveric renal transplant from an unrelated donor. Xenograft: A graft between individuals of different species, e.g. a monkey kidney into a human. Orthotopic transplant: A transplant grafted into its normal site. Heterotopic transplant: A transplant placed into an abnormal site, e.g. kidney transplantation into the iliac fossa and not the loin. Immunological basis of allograft rejection Tissue and organs transplanted between genetically disparate individuals are, in the absence of immunosuppressive therapy, rejected within a few days of grafting. The rejected graft becomes heavily infiltrated by lymphocytes and monocytes, suggesting that cell mediated responses rather than antibodies are involved in graft rejection. The T lymphocytes plays an essential role in graft rejection is clearly shown by the inability of congenitally athymic or neonatally thymectomized animals to reject an allograft. Restoring such animals with normal T cells readily restores their ability to reject a graft. Three main categories of graft rejection are recognized: Hyperacute rejection: This occurs almost immediately after transplantation. It is due to the presence in the recipient of preformed cytotoxic antibodies because of previous sensitization to graft antigens by blood transfusion or an earlier graft. The complement fixing antibodies rapidly damage the graft vasculature with platelet aggregation, thrombosis and infarction. Fortunately careful pre-transplant screening has almost eliminated this type of rejection. Acute rejection: This is due to classical cellular immune mechanisms and occurs to some extent during the first few weeks of most transplants. The graft becomes heavily infiltrated with T cells, B cells, macrophages and NK cells. Both cytotoxic T cell lysis and delayed type hypersensitivity responses appear to be capable of causing graft damage, but in the majority of cases any such rejection episode can be reversed by the immunosuppressive therapy. Chronic rejection: If a graft is not acutely rejected within the first few weeks, the chances of long term survival are much improved. Nevertheless, a small proportion of grafts steadily succumb to chronic rejection many months after transplantation. Humeral responses appear to be involved in late chronic rejection. The glomerular capillaries become coated with antibodies and gradual fibrosis and loss of renal function occur. Tissue typing Close matching of donor and recipient with respect to transplantation antigens is an essential requirement for bone marrow transplantation to avoid rapid graft rejection or graftversus-host disease. By contrast, matching for corneal graft is unnecessary since the cornea appears to be an immunologically privileged site. In renal transplantation (and possibly for other transplanted organs) attempts to match the tissue types of donor and recipient produce a definite increase in graft survival. The first requirement in organ matching is to match ABO blood group antigens, since these antigens are expressed not only on red blood cells but on most cells of the body, and if mismatched will elicit a strong immune response in the recipient due to the presence of naturally occurring antibodies to the ABO antigens. Tissue typing is employed to identify an individual's leukocyte antigens or HLA type. These transplantation antigens are expressed on most cells of the body and they are coded for by a cluster of genes---- the major histocompatibility complex (MHC)--- situated on chromosome 6. The MHC codes for the alleles of the HLA-A, HLA-B and HLA-C loci (class I MHC antigens), and for the alleles of the HLA-DR, -DP and –DQ loci (class II MHC antigens). HLA-A and -B loci and in particular the DR loci are considered to code for the most important transplant antigens. To date, there are 20 well defined A locus alleles, 33 B locus alleles and 10 DR alleles. An individual possesses 2 alleles for each locus (one inherited from each parent), but may be homozygous at one or more loci. Logistically, for the majority of renal transplants, perfect matching of donor and recipient at all loci is not possible. In practice, it is common to attempt to match both or at least one of the DR loci (since there are fewer DR types than HLA-A and-B types) and there after, where possible the HLA-A and-B loci. Retrospective studies after renal transplantation show that with an increasing number of HLA-DR, -A and-B mismatches there is a progressive decrease in the graft survival rate. An individual's tissue type is determined in the laboratory from a preparation of peripheral blood leucocytes; these are tested serologically, using typing anti-sera. In addition to tissue typing, as part of the organ matching procedure it is essential to perform a cross match prior to transplantation, using recipient serum and donor lymphoid cells, in order to exclude the presence in the recipient of pre-formed cytotoxic antibodies if these antibodies are present it could result in hyperacute rejection of the graft. It should be emphasized that despite a complete match of the HLA-A, -B and –DR locus antigens, rejection episodes may still occur in response to minor histocompatability antigen differences, but these tend to be amenable to immunosuppressive therapy. Methods of preventing rejection Although tissue typing reduces the chances of graft rejection, it is essential to give immunosuppressive drugs after organ grafting and these have to be continued indefinitely to prevent rejection. Until recently the most widely used immunosuppressive regimen was a combination of azathiobrine and prednezolone which act by non-specifically ablating the immune response. Recently, cyclosporin A, a newer drug, has been used increasingly. This important drug is a metabolite of a fungus and its action is more specifically directed at lymphoid cells than conventional immunosuppressive drugs. cyclosporin A exerts its powerful immunosuppressive effect mainly through its action on T cell activation, preventing the development of cytotoxic T cells. Unfortunately, its side effects include nephrotoxicity and hepatotoxicity. If signs of rejection occur, despite the presence of the above immunosuppressive drugs, then additional immunosuppressive measures may be introduced. These include the use of antilymphocyte serum (ALS). This serum is raised by injecting an animal (commonly a rabbit or horse) with human lymphocytes. When the globulin fraction of the resulting antiserum is injected into the transplant recipient, it selectively destroys lymphocytes. A drawback of this treatment is that it may cause hypersensitivity reactions to the injected animal proteins. Recently the administration of monoclonal antibodies directed at T lymphocytes has also been tried with some success (OKT3). After injection, the monoclonal antibody binds to T cells which are rapidly depleted (probably by opsonisation) from the body. Both of the above agents may be successful in reversing a graft rejection episode. Tow other supplementary immunosuppressive treatments which have been tried are thoracic duct drainage and total lymphoid irradiation. Although both methods effectively deplete the body of circulating lymphocytes, they are serious undertaking and neither has been widely adopted in clinical practice. Complications of immunosuppression All immunosuppressive regimens used in organ transplantation increase the risk of infection and malignancy. Transplant recipients using immunosuppressive therapy are at high risk from opportunistic infection, especially by viruses. Opportunistic infection is a potential problem in all transplant recipients, but those receiving aggressive immunosuppressive therapy after liver, heart, lung and small intestinal transplantation are most at risk. Chemoprophylaxis is important in high-risk recipient. The risk of bacterial infection is higher during the first month after transplantation. It is a common practice to use a prophylactic antibiotic in such patients as any other patient going to have a major surgery to cover the per operative period. This is especially applicable in patients who are ill before surgery. The risk of viral infection is higher during the first 6 months after transplantation and the major common problem is CMV infection that may present as pneumonia, gastrointestinal disease, hepatitis, retinitis or encephalitis. Now, it is common practice to use a prophylaxis in the form of passive immunization (hyperimmune immunoglobulin) or administration of antiviral agents in the form of aciclovir. Protozoal infection is serious and commonly affects the recipient within the first 6 months after transplantation, the most common organism is pneumocystis carinii and the best prophylaxis is high doses of co-trimoxazole for 6 months after transplantation. Fungal infections not common after renal transplantation but more common after other solid organ transplantation. It occurs within the first 3 months after transplantation and the most common organisms are Candida or Aspergillus, if not early diagnosed and aggressively treated it might be fatal. Malignancies especially those viral related are common with immunosuppressive therapy after transplantation and it might arise up to 20 years after transplantation. The most commonly recorded malignancy with immunosuppressive therapy are skin malignancy like squamous cell carcinoma and basal cell carcinoma, also nonhodgikin's lymphoma is common whether affecting the lymph nodes or extralymphatic. Example of transplantation processes Kidney transplantation Renal transplantation is the preferred treatment for many patients with end-stage renal disease because it provides a better quality of life for them than dialysis. Transplantation releases patients from the dietary and fluid restriction of dialysis and the physical constraints imposed by the need to dialyse it is also more cost-effective than dialysis. The transplant kidney is placed in the retroperitoneal position, leaving the native kidneys in place. After induction of general anesthesia a central venous line and a urinary catheter are inserted. It is helpful to distend the bladder with saline containing methylene blue to allow to be identified with certainty prior to ureteric implantation. A curved incision is made in the lower abdomen and after dividing the muscles of the abdominal wall, the peritoneum is swept upward to expose the ilial vessels. These are dissected free so that they can be controlled with vascular clamps. The donor renal vein is then anastomosed end to side to the external iliac vein. The donor renal artery is anastomosed end to end to the internal iliac artery. While the vascular anastomosis is being under taken the kidney is kept cold by application of ice. Following completion of the venous and arterial anastomosis the vascular clamps are removed and the kidney is allowed to reperfuse with blood. The ureter, which is kept reasonably short to avoid the risk of distal ischemia, is then anastomosed to the bladder in an antireflux manner. Before closing the transplant wound it is important to ensure that the kidney is lying in a satisfactory position without kinking or torsion of the vessels. Technical complications: Renal artery thrombosis occurs in 1% of cases and renal vein thrombosis occurs in 5% of cases. The incidence might be minimized by using small doses of heparin; the diagnosis is done by the appetence of sudden onset of pain with swelling at the site of the transplant and confirmed by Doppler study. The treatment is nephrectomy. Renal artery stenosis presents late, even years after transplantation (about 10% of cases), presented with deterioration of the kidney function and hypertension, usually treated now with angioplasty. Ureteric leak occurs in up to 10% of cases and can be avoided with stenting and can be treated with reimplantation. Early or late ureteric obstruction can be presented with deterioration of the kidney function and diagnosed by ultrasonography; surgical correction is needed although trials of balloon dilatation may be of help. Outcome after transplantation: The half life of the transplanted kidney of living related donor is much better than that of living unrelated which is again much better than the cadaveric one. The range is 7 to 24 years. Pancreas transplantation Successful pancreas transplantation restores normal control of glucose metabolism and obviates the need for insulin therapy in patients with diabetes mellitus. Improved control of blood glucose levels in diabetes undoubtedly reduces the risk of secondary complications such as retinopathy, peripheral vascular disease and nephropathy. However in considering the indications for pancreas transplantation, these advantages have to be weighted carefully against the risks posed both by the transplant procedure itself and the immunosuppressive therapy required to prevent graft rejection. Careful patient selection is essential to avoid excessive mortality and morbidity. The procedure is usually reserved for those patients with type I diabetes who are relatively young (under the age of 50 years0 and do not have advanced coronary artery disease or peripheral vascular disease. Echocardiography and coronary angiography are mandatory during assessment of recipient suitability for transplantation. Most centers now perform transplantation of the whole pancreas together with a segment from the duodenum. The pancreas graft is placed intraperitonially in the pelvis and the donor vessels of the graft are anastomosed to the recipient iliac vessels and the exocrine secretions are dealt with by anastomosing the graft duodenum to either the bladder (urinary drainage) or the small bowel (enteric drainage).although urinary drainage has a higher incidence of urinary tract infections but it has the advantage that urinary amylase levels can be used to monitor graft rejection. The results of pancreas transplantation have improved significantly over the last decade. The one year patient survival rate is greater than 90%. The treatment of diabetes by transplantation of isolated islet cells injected into the portal vein in the liver is under trial but the results now is not that good as the number of the cells needed for this is very high, the rejection rate is very high and the use of capsules of semipermiable membranes containing the cells is under trial. Liver transplantation The indication for liver transplantation falls into four groups: Chronic cirrhosis. Acute fulminant liver failure. Metabolic liver disease. Primary hepatic malignancy. A transverse abdominal incision with a midline extension is made and the diseased liver mobilized. Because of portal hypertension the recipient hepatectomy is often the most difficult part of the operation, especially if there has been previous surgery in the region. The common bile duct is divided as are the right and left hepatic arteries. The inferior vena cava is clamped and divided allowing the recipient liver to be removed. Occlusion of the vena cava and portal vein results in a reduction in cardiac output and may necessitate the use of veno-venous bypass. The bypass delivers blood from the vena cava and portal vein to the heart via a canula inserted in the axillary vein or internal jugular vein. After putting the donor liver in position, the supra and infra hepatic caval anastomosis are performed. The portal vein and hepatic arterial anastomosis are then performed and the graft is reperfused. Finally, biliary drainage is re-established, usually by a duct to duct anastomosis (without the use of a T tube), it may be necessary, for example in recipients with biliary atresia or sclerosing cholangitis, to reconstruct the biliary drainage by a bile duct to Roux loop anastomosis. Technical complications 1-Haemorrhage: correction of coagulopathy and meticulous haemostasis is mandatory. 2-Heptic artery thrombosis may result spontaneously or as a result of rejection and diagnosed by Doppler study to evaluate the deterioration in liver function or biliary leak, this is usually need retransplantation. Portal vein thrombosis is less frequent and usually dose not need retransplantation. 3-Biliary leak is now less frequent and usually managed by endoscopic dilatation or stenting. The outcome of liver transplantation for a chronic liver disease is much better than the outcome after transplant for acute liver failure. The outcome after primary liver malignancy is good but the transplant after a hepatitis C or hepatitis B is usually followed by recurrence of the viral infection. Small bowel transplantation Small bowel transplantation is a treatment option for patients with intestinal failure as defined by the loss of intestinal function to the extent that long term parental nutrition is required. Intestinal failure may be due to: Intestinal atresia. Necrotizing enterocolitis. Volvulus. Disorders of motility. Mesenteric infarction. Crohn's disease. Trauma. Desmoid tumors. The superior mesenteric artery of the graft (it is not advisable to include the ascending colon in the graft) is anastomosed to the recipient aorta, and the superior mesenteric vein is anastomosed to the inferior vena cava or to the side of the portal vein. The proximal end of the small bowel graft is anastomosed to the recipient jejunum or duodenum. The distal end of the graft is anastomosed to the side of the colon. The one year graft survival rate is about 60% and after three years the graft survival rate is around 40%. Heart Transplantation Transplantation is considered only in patients with end stage heart disease which has failed to respond to all other conventional therapy and where predicted survival without transplantation is only six to twelve months. A median sternotomy is performed and the patient is given systemic heparin, placed on cardiopulmonary bypass and cooled to 280C. After cross clamping the aorta, the recipient heart is excised at the mid-atrial level. The donor heart is then removed from ice and the left atrium is then opened by making incisions in the posterior wall between the orifices of the pulmonary veins to create an atrial cuff. The left and then the right atrial anastomosis are performed and the aortic and pulmonary arterial anastomosis are then completed. The patient is then rewarmed and weaned from cardiopulmonary bypass. One- and five- year graft survival after heart transplantation is around 85 and 70% respectively. Bone marrow transplantation This offers a chance of cure in an increasing number of patients with otherwise fatal malignant and non-malignant conditions. It is most often used in the management of haematological malignancy. It also has a role in non-malignant diseases such as sever combined immune deficiency; sever aplastic anaemia and thalassaemia major. In essence, the patient is given a very high dose of chemotherapy or total body irradiation to destroy completely the malignancy and also the patient's own lymphoid tissue and bone marrow which would otherwise rapidly reject the graft. Bone marrow from a well matched donor, invariably a sibling since this offers the best chance of good match, is then transplanted at the appropriate time and the transplant recolonizes the recipient's bone marrow. Although the technique is relatively simple, marrow grafting is a hazardous process. Complications related to graft rejection, infection and bleeding may be difficult to control and even when the donor is an HLA identical sibling and in the presence of immunosuppressive drugs, graft-versus-host reaction is still common. The risk of graft-versus-host reaction can be reduced if T cells can be eliminated from the donor marrow prior to transplantation. A promising way of achieving this involves incubating the harvested marrow with anti-T-cell monoclonal antibodies, either with complement or bound to toxin, in an attempt to destroy any T cells. Questions: 1- Discuss immunological basis of allograft rejection and methods of preventing rejection? 2- Discuss indications and technical complications of liver transplantation?