Investigation of some properties of a pair of cis- trans
advertisement

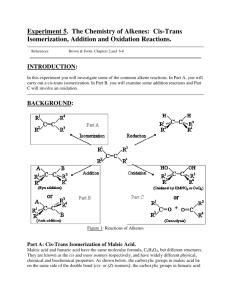
OUR LADY OF THE ROSARY COLLEGE CHEMISTRY PRACTICAL MANUAL Advanced Level, F.6 Experiment should be carried out under the supervision of a Chemistry teacher Safety precaution must be noted. Division of work: individual Investigation of some properties of a pair of cis- trans- isomer Maleic acid and fumaric acid are geometric isomers. Both of them have the same molecular formula C4H4O4. They have similar physical and chemical properties, however, each of these isomers also has its own distinctive properties such as melting points, solubility, density and stability. In this practical, we shall investigate the above properties of the cis- and trans- isomers. In addition, we can identify which one is the tans-isomer by its distinctive properties. Chemicals Magnesium ribbon and sand paper, bromine water, 0.50 M sodium hydroxide, sodium carbonate solid, phenolphthalein indicator, 'maleic acid' and 'fumaric acid' [both have been prepared in the previous practical] Additional Apparatus Burette(x8), pH meter (x2), triple beam balance(x3) Procedure 1. 2. 3. 4. 5. Compare the solubility in water: place 1 spatula (about 0.5 g) of one of the acids into 10 cm3 of water in a test-tube, shake to help dissolving, and keep the solution or Test 3. Compare the melting points: Using the electrical melting point apparatus, measure the melting points of the two isomers. (Melting point of fumaric acid is higher than 180 ℃. Your teach will give this value to you as a reference value.) Compare the acid strength: Add about 10 cm3 of distilled water into solution from step (1). Divide the solution into 3 portions. One for each of the following tests: (a) Measure the pH of the solution. (b) Place a strip of magnesium ribbon into the solution and observe. (c) Add a small amount (size of a pea) of solid sodium carbonate. Reaction with bromine water: Suspend several pieces (about 0.1 g) of one of the acids in about 5 cm3 of water. Add 3 drops of bromine water to the resulting solution/suspension. Shake and observe. Repeat for another acid. Reaction with alkali: Weigh about 1.00 g of one of the acids. Transfer the acid to 25 cm3 of distilled water in a titration conical flask. Shake the mixture for a while. The acids may not be soluble in water readily. Titrate against the standard 0.50 M NaOH solution provided. Appendix: Use of Melting point apparatus One end of capillary tube is sealed. Insert the open end into the dry sample. Allow the capillary tube to fall freely in a 1 m glass tube such that the dry sample falls down to the bottom of the tube. Repeat this practice until the height of the sample in the capillary tube is about 0.5 cm. You then place the capillary tube into one of the 3 holes of the melting point apparatus (If temperature of the apparatus is higher than 80 ℃, then let it cools down to this temperature) Turn on the power supply (turn on both switches) and note the appearance of the solid inside the capillary tube. If a minute solid melts, note the reading of the thermometer immediately and it gives you the melting point of this sample. Turn off the apparatus. Data and Results Maleic acid Fumaric acid 1.Solubility in water 2.pH value 3.Reaction with magnesium 4.Reaction with bromine Titration data Final burette reading Initial burette reading Titre used Guided Questions 1. 2. 3. 4. 5. 6. 7. 8. What do each of the following tests contribute to your knowledge of the structure of each isomer? (Simple answer is required.) (a) The reaction with magnesium (b) The reaction with sodium carbonate. Consider the structures of the two acids, try to account for the observed differences in solubilities. Account for the difference in the melting points of maleic acid and fumaric acid. Explain the difference in pH value for maleic acid and fumaric acid. Give an equation for the neutralization reaction between sodium hydroxide and fumaric acid. By using the titration data, calculate number of displacable hydrogen per one mole of (1) maleic acid and (2) fumaric acid. Give structure of reaction product for the reaction between maleic acid and bromine. Maleic acid may lose a molecule of water from each molecule of acid when its two carboxylic acid groups react to form an anhydride. Which structural isomer, cis- or trans- do you predict it is? Explain your prediction.