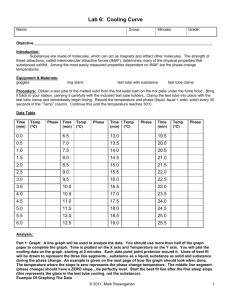
Melting Temperature of a Solid with Data

Unit 1: Matter and Energy
Name: ______________________________
Melting Temperature of a Solid Date: ________________ Class: _________
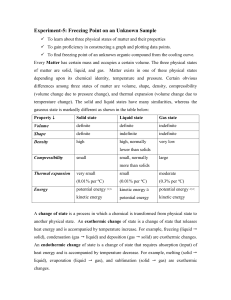
A characteristic physical property of a pure substance is its melting and freezing temperature. In this lab you will investigate the melting and freezing behavior of an unknown pure substance and use those data to identify the substance.
Procedure:
Part I: Cooling Behavior of a Pure Substance
NOTE: All temperatures should be read to the nearest 0.1ºC.
1.
Place approximately 350 mL of tap water into a 400 mL beaker. DO NOT USE THIS YET.
2.
When you are ready to record temperatures, obtain a test tube containing the liquefied substance from the beaker in the fume hood.
3.
As soon as you obtain the test tube, IMMEDIATELY record the temperature at time = 0.
4.
Clamp the test tube to a ring stand and allow it to cool IN THE AIR. Gently move the thermometer up and down in the liquid until it has solidified. LEAVE THE THERMOMETER IN THE SOLID!
5.
Record the temperature of the substance every 30 seconds, noting on your paper when solidification begins and ends.
6.
When the substance has cooled to 50ºC, lower the test tube into the water.
7.
Continue to record temperatures until the temperature is approximately 25ºC.
Part II: Warming Behavior of a Pure Substance
1.
Raise your test tube (clamped to ring stand) out of the beaker of water.
2.
Place a thermometer into your beaker of water.
3.
Heat the beaker of water using a Bunsen burner until it reaches about 65ººC.
4.
Turn off the burner, but be prepared to relight it. THE WATER MUST BE KEPT BETWEEN 60ºC
AND 65ºC.
5.
Lower your test tube into the water and immediately record the temperature of the SOLID.
6.
Record the temperature of the substance every 30 seconds, noting on your paper when melting begins and ends.
7.
As soon as the solid becomes free from the walls of the test tube, gently move the thermometer up and down.
8.
Continue recording temperatures until the substance reaches 60-65ºC.
Part III: Analysis
1.
Create a Cooling Curve, using the data collected in part 1. a.
Label each axis. b.
Give your graph a title. c.
Identify the Freezing Point of the substance. d.
Mark each section of the graph with the correct type of energy present. e.
2.
Create a Heating Curve, using the data collected in part 2. a.
Label each axis. b.
Give your graph a title. c.
Identify the Freezing Point of the substance. d.
Mark each section of the graph with the correct type of energy present.
3.
What is the relationship between the freezing point and the melting point of a pure substance?
4.
Use the following list of substances and their melting points to identify your substance?
Name
Mesityl Bromide
Diisopropanolamine
Dodecanoic acid
Thymol
Dibromoacetic acid
Paradichlorobenzine
Dibromoaniline
Procaine
Melting Point
40°C
42°C
44°C
50°C
48°C
53°C
57°C
61°C
5.
Conclusion:
DATA FOR LAB:
Time (s)
0
30
60
90
120
150
180
210
240
270
300
Part 2 Melting Temperature
(degrees C)
35
45
50
52
52
52
53
55
60
71
Part 1: Freezing Temperature
(degrees C)
70
60
53
53
52
52
50
50
50
43
33