CHEMISTRY WORKSHEET ON LIGHT
advertisement
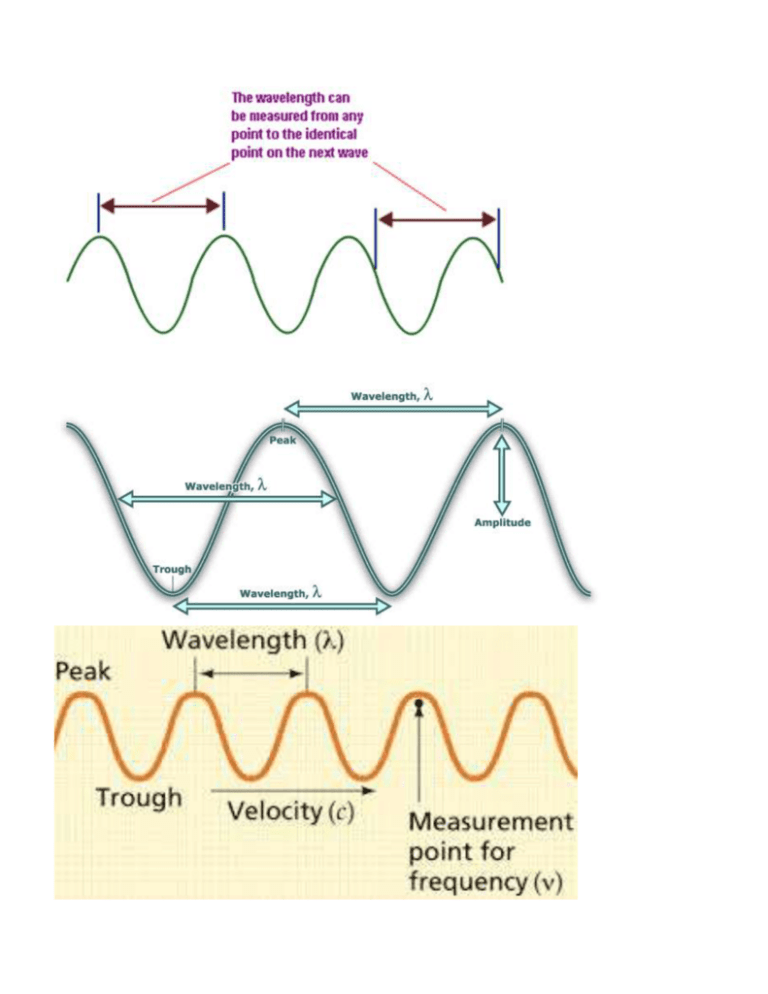
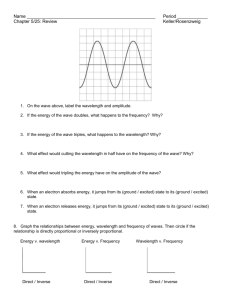
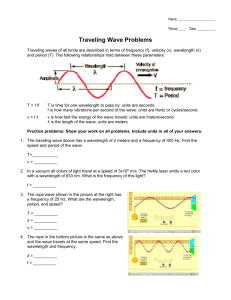
Name:__________________Hour: LIGHT WORKSHEET #1 Solve the following problems on a separate sheet of paper. 1. (a) Write the mathematical equation showing the relationship between wavelength and frequency for an electromagnetic wave. (b) Indicate how the variables are related to one another. 2. (a) Write the mathematical equation showing the relationship between energy and frequency for an electromagnetic wave (b) Indicate how the variables are related to one another. 3. Indicate how the variables wavelength and energy are related to one another for an electromagnetic wave. 4. The wave below is NOT an electromagnetic wave so we don’t know for certain it goes at the speed of light! You can’t use the equations, These questions are testing your understanding of the definitions of wavelength and frequency and you must use the information given in the question and the picture to figure them out. (a) Use the diagram below and the definition of wavelength to find the wavelength of the wave. (b) Use the definition of frequency and assume the entire wave below traveled for 0.017 seconds to find the frequency. (c) Use the wavelength and frequency to find the velocity in m/sec. 5.24 m 5. Given an electromagnetic wavelength of 5.7 X 10-5 m/cyc, calculate the (a) frequency and (b) energy of this wave. 4.0 4.4 4.9 5.5 5.8 6.3 7.0 | | | | | | -7 | | | violet | | | blue | | | ( in m X 10 ) | | green |yellow| | | orange red 6. The frequency of a light wave equals 6.0 X 1014 cyc/sec. Calculate the (a) wavelength (b) color and (c) energy. 7. Given light energy of 3.2 X 10-12 erg (1erg = 1 x10-7 J), calculate the (a) frequency (b) wavelength and (c) color. 8. The-7energy of a certain type of electromagnetic radiation is 1.88 X 10-16 erg (1erg = 1 x10 J). Calculate the (a) frequency (b) wavelength and (c) type of radiation that has this energy. -13 10 -11 -9 10 -7 10 -5 10 -3 10 -1 10 10 1 10 ( in m) | | | Gamma | radiation | | 9. | | | | X-rays | | | | Ultra Violet light | | | | |V| |i| |s| | | Infrared light | | | | | | Micro waves | | | | | TV & radio | signals | The energy of some electromagnetic wave equals 2.9 X 10-8 erg (1erg = 1 x10-7 J). Calculate the (a) frequency (b) wavelength and (c) type of radiation of this wave. 10. When the soprano hits high C in the mad scene of the opera “Lucia di Lammermoor”, there is an appreciative sigh throughout the audience. High C on the musical scale has a frequency of 1024 cyc/sec. Find the wavelength in of the high C sound wave. (The velocity of a sound wave is equal to 331 meters/sec. DO NOT USE c FOR THE VELOCITY….this is a sound wave, not light.) 11. How long (in sec) would it take a radio signal wave to travel from the Earth to the planet Mars, a distance of approximately 8.0 X 107 km? (Remember that a radio signal is a form of electromagnetic radiation. Use c for the velocity. Use dimensional analysis. Convert the km to cm and then use c as a conversion factor. 12. Joan is wearing a blue shirt, Sheila is wearing a green shirt and Roslyn is wearing a red shirt. Which girl is wearing the shirt that is giving off the most energy in terms of light energy being reflected? Use the color chart to justify your answer. 13. Given an electromagnetic radiation energy value of 5.6 x 10-13 erg (1erg = 1 x10-7 J), find the (a) frequency, (b) wavelength and (c) type of radiation associated with this value. 14. Given a light of frequency of 5.35 x 1014 cyc/sec, find the (a) wavelength, (b) color and (c) energy associated with this value.