Chapter 7 光谱导论
advertisement
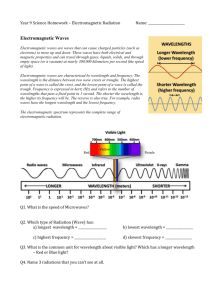
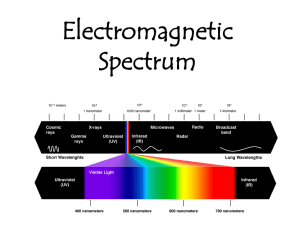
Introduction to Spectrometry 光谱分析导论 (P201-203;270-272) Spectroscopy is the use of the absorption, emission, or scattering of electromagnetic radiation by matter to qualitatively or quantitatively study the matter or to study physical processes. The matter can be atoms, molecules, atomic or molecular ions, or solids. 光谱分析方法的分类 分子光谱 原子光谱 紫外可见法 原子吸收法 红外法 光谱分析法 核磁法 原子发射法 荧光法 8.1 Electromagnetic Radiation(电磁辐射) and Electromagnetic Spectrum(波谱) 光 是 一 种 电 磁 辐 射 ( 电 磁 波 、 横 波 ) 电场 磁场 传播方向 粒子性:光是由具有能量的光子组成,光子具 有质量和动能,每个光子能量 E h Unit hc Relation to Meter meter (m) millimeter (mm) 1mm = 10-3m micrometer (μm) 1μm = 10-6m nanometer (nm) 1nm = 10-9m Angstrom (Å) 1 Å = 10-10m E2 E1 E hc υ Sometimes it is convenient to label the radiation with the inverse(倒数)of the wavelength. The inverse of centimeters (cm-1) is called the wave number(波数), and the unit is used most often in infrared spectrometry. A measurement made in wave numbers is often denoted by υ . Then, the photon energy is the product of hc and the wave number. That is, 电磁波谱(electromagnetic spectrum) Electromagnetic -ray -ray ultraviolet visible infrared microwave radio Wave Wavelength (nm) (nm) 10-3 to 10-2 10-2 To 10 (nm) (nm) (m) (cm) (cm) 10~380 380~780 0.78~100 0.1~1 10~ Example 8.1 What is the energy per photon of the sodium D line ( 589nm )? Solution The energy per photon of the sodium D line is E hc ( 6 . 626 10 34 J s ) 3 10 m s 589 10 8 9 m 1 3 . 37 10 19 J 8.2 Interaction of Electromagnetic Radiation with Matter Rotational movement 旋转运动 Vibrational movement 振动运动 Electronic movement 电子运动 Energy: discrete levels (quantization 量子化) When an atom, ion or molecule changes energy state, it absorbs or emits energy equal to the energy difference E = E1 - E0 Absorption EM ground state 基态(E0) Transition 跃迁 excited state 激发态(Ei) The wavelength or frequency of radiation absorbed or emitted during a transition proportional to E Transition: EM: E=h equal to ΔE = E1 - E0 Rotational Transition 转动跃迁 (0.005~0.050eV) (100m~10cm; far-infrared; microwave; line spectrum) Vibrational Transition 振动跃迁 (0.05~1eV) (2.5~15; 0.78~2.5m; mid-infrared; near-infrared) Electronic Transition 电子跃迁 (1~20eV) (10~780nm; far-UV; near-UV; visible) With molecules, we not only have electronic but vibration and rotational sub-levels. Interactions with other molecules and a solvent will also have an effect. These result in “band” spectra. In atoms, there is a type of movement, i.e. electronic movement. 原 子 光 谱 分子光谱