Activity: Discovering the Salt Concentration of Potatoes
advertisement

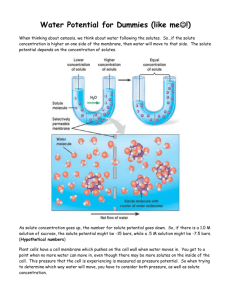
Activity: Discovering the Salt Concentration of Potatoes Problem: What is the salt concentration of potatoes? Purpose (1 mark) Materials: -test tubes -10mL graduated cylinder -2 100mL beakers -2mL, 5mL, 10mL pipettes -test tube rack -potato -1 mol/L (= 1M) sucrose solution -distilled water -Sharpie marker for test tubes -grid paper (for graph) -knife -ruler -paper towels -electronic balance -tweezers Procedure: 1. Label 6 test tubes, #1 to #6, and place in test tube rack. 2. Using a pipette, add the correct amount of water to each test tube and then the correct amount of 1 mol/L sucrose solution to prepare the proper solute concentration for each tube: Test Tube Number Volume of Water (mL) Volume of 1 mol/L Sucrose Solution (mL) Solute Concentration (mol/L) 1 10 0 0 2 8 2 0.2 3 6 4 0.4 4 4 6 0.6 5 2 8 0.8 6 1 10 1.0 3. Use knife to cut 6 equal sections of potato measuring 1cm x 1cm x 4cm. 4. Rinse potato sections with distilled water and blot dry with a paper towel. 5. Determine the mass of each section of potato and record. Place each section into one of each of the six test tubes, keeping track of which section is in which tube. 6. After 24-48 hours, remove each potato section gently with tweezers and gently blot it dry. Record the final mass of each potato section in your table. 7. Calculate the % change in mass for each potato section and record in your table: % Change in Mass = Final Mass – Initial Mass x 100% Initial Mass 8. On grid paper, plot the percent change in mass vs. solute concentration. Include both negative and positive numbers (if necessary) on your y-axis. Make a straight line of best fit through your data points. Observations: (one data table with descriptive title, and one graph with all appropriate labels/title) Discussion: 1. Determine the solute concentration of the potatoes by interpolation with your graph (hint: what % change in mass would be expected if the potato had the same solute concentration of the surrounding solution?). 2. Indicate on your graph the solutions that were either hypertonic or hypotonic to the potato cytoplasm. 3. Explain why some potato sections lost mass while others gained mass. 4. What can happen to a person if intravenous fluids are not of the same solute concentration as blood? Explain both cases (intravenous fluids being hypertonic or hypotonic). Give 2 common examples of when intravenous fluids are administered to a patient. 5. Road salt spilled on grass often kills the grass. Explain why, using the knowledge from this investigation. Sources of Error (give at least 2, not human error) Conclusion (1 mark)