Renal Allograft Dysfunction

A
PPROACH TO
R
ENAL
A
LLOGRAFT
D
YSFUNCTION
Allen Repp, M.D. October 11, 2002
Description
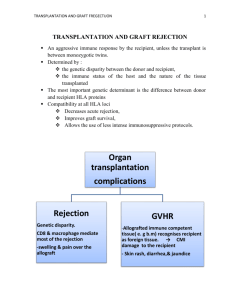
Renal allograft dysfunction is the most common complication of renal transplantation
Risk factors for diminished allograft survival include prior cadaveric transplantation, evidence of prior sensitization, higher degrees of HLA mismatch, donor ages <5 or >60, occurrence of delayed graft function, allograft dysfunction at discharge from transplantation, and episodes of acute rejection
1 year survival of 1 st allograft is approximately 86% for cadaveric and 92% for living-related
(although there is consider variability between transplant centers)
Time after transplantation provides the framework for consideration of the causes of allograft dysfunction, since the prevailing causes vary with the time after transplantation.
Immediate Post-Transplantation (1 week)
Delayed graft function (DGF) represents renal failure persisting after transplantation and is usually defined as oliguria or requirement for dialysis in the first week of transplantation
Major Causes:
Post-ischemic acute tubular necrosis (ATN) = most common cause of delayed graft function
Risk factors: cold ischemia time > 24 hours with cyclosporine induction, prior sensitization, and use of bioincompatible membranes and/or ultrafiltration within 24 hours of transplant
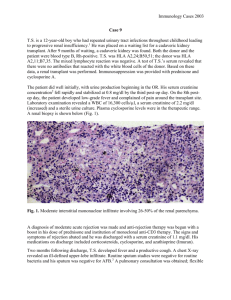
Biopsy: sloughed and swollen tubular epithelial cells with scalloping of epithelial cells
Hyperacute rejection = rare, and usually avoidable cause of immediate allograft dysfunction
Often diagnosed intra-operatively when the transplant kidney becomes mottled and cyanotic, with absence of blood flow on Doppler
Usually caused by unrecognized ABO incompatibility or anti-HLA class I antibodies
(positive T cell cross match)
Biopsy: intrarenal coagulopathy with thrombi of small arteries and glomeruli, and renal cortical necrosis
Accelerated rejection = episode of rejection occurring 2-5 days post-transplant
Can occur with or without delayed graft function
Attributed to prior sensitization to donor antigens or by anti-HLA class II antibodies
(positive B cell mismatch)
Biopsy: cellular rejection with interstitial infiltrate with mononuclear cells and eosinophils and disruption of basement membrane by infiltrating cells, and/or vascular rejection with capillary endothelial swelling, arteriolar fibrinoid necrosis, and fibrin thrombi in glomerular capillaries
Atheroembolic disease and thrombosis
Atheroemboli can derive from donor’s aorta at time of organ procurement or from recipients vascular system at time of transplantation
Thrombosis can occur in setting of graft rejection (aka secondary thrombosis), or rarely in the absence of rejection (aka primary thrombosis), associated with increased donor age, longer cold ischemia time, presence of anti-phospholipid antibodies, and hypersensitization
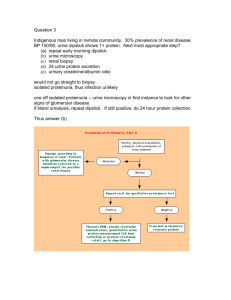
Evaluation:
Perform ultrasound to assess urinary leak or obstruction
In high risk patients proceed to biopsy quickly (day 3-5) to evaluate for rejection
In low risk patients proceed to biopsy on day 7-10
Treatment is based on biopsy findings -- pulse dose methylprednisolone for predominantly cellular rejection or OKT3 for predominantly vascular rejection
If no evidence of rejection on initial biopsy but delayed graft function persists, repeat biopsy is warranted
Beth Israel Deaconess Medical Center Residents’ Report
Early Post-Transplantation (1-12 Weeks)
Initial allograft function followed by early renal insufficiency
Major Causes:
Acute rejection = most common cause of allograft dysfunction during the early posttransplant period
Once occurred in 50-60% renal transplant patients, now estimated to occur in 15-25%
Heralded by diminished urine output and increasing blood pressure
Biopsy: similar to hyperacute rejection with attempts made to distinguish cellular rejection from vascular rejection
Cyclosporine or tacrolimus (FK506) toxicity
Both of these calcineurin inhibitors are nephrotoxic through a variety of mechanisms and can lead to oliguric ATN, HUS-like syndrome (rare), and chronic progressive renal dz
Important to distinguish from acute rejection, as the management is obviously completely different
Urinary tract obstruction
Prerenal azotemia secondary to volume depletion
Infection – Polyoma virus (BK type) interstitial nephritis and CMV-mediated renal disease
Recurrence of primary disease such as focal glomerulosclerosis, HUS-TTP, or anti-GBM disease
Drug induced acute interstitial nephritis
Evaluation:
Check UA for evidence of recurrent disease or infection
Check plasma cyclosporine levels. If elevated (>150 ng/mL), decrease dose and re-evaluate in 1-2 days
Renal US to exclude obstruction or urinary leak
Biopsy to evaluate for acute rejection. If rejection present, treat with corticosteroids
Late Acute Dysfunction (>12 weeks)
Acute allograft dysfunction occurring more than 3 months post transplant
Major Causes:
Prerenal azotemia secondary to volume depletion
Cyclosporine or tacrolimus toxicity
Acute rejection (consider in the setting of decreasing immunosuppressive doses or medical non-compliance)
Urinary tract obstruction
Recurrence of primary disease
Renal artery stenosis (associated with HTN)
Infections – CMV or human polyoma virus type BK associated interstitial nephritis
Evaluation:
Replete intravascular volume and repeat creatinine measurement
Check plasma cyclosporine levels. If elevated (>150 ng/mL), decrease dose and re-evaluate in 1-2 days
Renal US to exclude obstruction or urinary leak
Renal biopsy
Late Chronic Dysfunction
Slowly progressive renal insufficiency over a period of months to years
Major Causes:
Chronic allograft nephropathy / chronic rejection
Cyclosporine nephrotoxicity
Hypertensive nephrosclerosis
Infections – CMV and polyomavirus
Recurrence of primary disease – e.g., focal glomerulosclerosis, membranoproliferative GN,
IgA nephropathy, diabetic nephropathy
Evaluation:
Often difficult to determine cause of chronic allograft dysfunction, even with biopsy
No available treatment for chronic allograft nephropathy except for decreasing cyclosporine dosage as indicated and controlling blood pressure
Beth Israel Deaconess Medical Center Residents’ Report