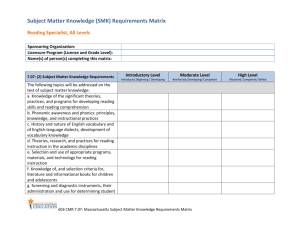
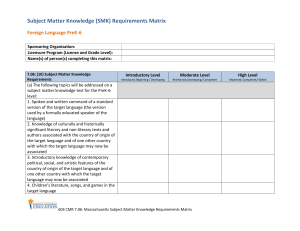
实验步骤: 1、Inoculate from an overnight grown in LB.从培养过夜的
advertisement

实验步骤: 1、Inoculate from an overnight grown in LB.从培养过夜的 LB 平板上挑取单菌落 。 2、Grow in 250 ml "SOB" at 18℃ until OD600 = 0.6.(0.3)接种于 250ml SOB,18 度培养 至 OD=0.6。 3、On ice for 10 minutes.菌液置冰上 10 分钟。 4、Spin at 2500 x g (5000 rpm in a Sorvall GSA or 3000 rpm in a Beckman J-6B centri fuge)for 10 min.at 4℃. b68 4 度 2500g 离心 10 分钟。 5、Resuspend cells gently in 80 ml of ice cold "TB".小心用 80ml 预冷 TB 重悬细胞。 6、On ice for 10 minutes.(30min)菌液置冰上 10 分钟。 7、Spin at 2500 x g (5000 rpm in a Sorvall GSA,5500 rpm in a Sorvall SS-34,or 3000 r pm in a Beckman J-6B centrifuge)for 10 min.at 4℃.4 度 2500g 离心 10 分钟。 8、Resuspend cells gently in 20 ml of ice cold "TB".小心用 20ml 预冷 TB 重悬细胞。 9、Add DMSO to a final concentration of 7%.加入 DMSO 至终浓度 7%。 10、Place on ice for 10 minutes.置冰上 10 分钟。 11、Aliquot into 1-2 ml and freeze in liquid nitrogen.分装,液氮速冻 。 12、Store in liquid nitrogen.冻存。 SOB Medium and TB (Transformation Buffer) 2% (w/v) bacto tryptone SOB 0.5% (w/v) yeast extract 10 mM Pipes 10 mM NaCl 55 mM MnCl2 2.5 mM KCl 15 mM CaCl2 10 mM MgCl2 10 mM MgSO4 TB 250 mM KCl 在加入 MnCl2 之前先用 5N KOH 调 pH 值到 6.7,adjust pH to 6.7 with 5N KOH prior pH 6.7 - 7.0 to adding the MnCl2 thanks to Markus Schneemann for the tip! Note: Competent cells are fragile (cell wall is thought to be weakened),therefore treat the cells g ently when preparing these cells.Do not vortex or pippette up and down to resuspend the cells.Do not spin the cells at too great a speed (spinning down at 5000g will cause some cells to lyse). Always keep the cells chilled when making competent cell.Do not let them warm up. Freezing the cells appear to make cells more competent. Some cell strains may work better than others (DH5alpha works well in my hand).Note al so that some cells (e.g.HB101)has greater recombination activity than others. This method doesn't appear to work with BL21,so just grow the cells at 30 or 37 ℃ whe n making BL21 competent cells.However,it h b68 as been suggested that the efficiency of BL21 prepared using Inoue method may be improved by treating it with DTT before fre ezing (add to 3.5% v/v of a 2.2M DTT,10mM KAc pH6 solution and incubate 10 minute s on ice). Heat shock time should be determined for different strains of cell.For DH5alpha or JM109 use 30-45 sec.For BL21 use 120 sec. Deactivate ligase prior to transformation.Ligase may reduce transformation efficiency. Diluting the ligation mixture (~5x)can also increase transformation efficiecy by reducing th e amount of reagents/contaminants that may affect transformation.Likewise it has been sug gested that phenol/chloroform treatment may also increase efficiency,but it is probably too much trouble to bother trying. The DNA added should not be more then 5% of the volume of competent cells used.The final DNA concentration should not exceed 5 ng/μl. The method above should give a transformation efficiency of more than 108 cfu per μg o f plasmid DNA (pUC or pBluescripts)with over 109 cfu possible. Transformation efficiency has a roughly inverse relationship with the size of plasmids.Cells with deoR mutaion (e.g.DH5alpha)can improved the transformation of large plasmid.Relax ed plasmids has ~3/4 of the transformation efficiency of supercoiled plasmid. 2 different plasmids can be transformed at the same time,or one after another.But they mu st be compatible (they cannot have the same replicon). For routine transformation whereby efficiency of transformation is of no import,some of th e steps may be shorten or omitted.For example,heat-shock step may be unnecessary and re covery incubation time at 37 ℃ can be reduced or omitted (but do note that this may de pends on the antibiotic used for selection- for ampicillin-type antibiotics the incubation tim e is not really that important,therefore you can plate the cells straight after heat-shock if you wish.for other antibiotics,however,the incubation time may be essential). Plating cells- dry 1.5% agar plates (exposed upside down)at 37 ℃ for 2-4 hours just befo re use,the plate should be able to soak up to 0.8-1 ml of media when plating.For blue-wh ite selection,it is not necessary to make X-gal plate,just add X-gal+ IPTG direct to cells,m ix and then plate. Detergents may be detrimental to the transformability of the competent cells,therefore the glassware used for making competent cells should not be washed with detergents.Polycarbo nate flask may also be used instead of glass flask.DMSO can dissolve polystyrene,therefor e use polypropylene tubes. When cloning difficult and less stable sequence (e.g palindrome,repeats,LTR sequences),it h elps to grow transform cells at lower temperatures (25-30 ℃ or room temperature)in very rich media (e.g.Terrific Broth).Also terminate growth before reaching late statio b68 nary growth phase when grown in liquid media (i.e harvest cells at OD550 between 1 and 2). Use of stabilizing strain is also useful. There are other methods of making competent cells- e.g.CaCl2 method,RbCl method which is more effective than CaCl2 method.Electroporation is supposed to give higher efficiency (up to 1010 transformants per μg plasmid claimed),but for the simple cloning that we do, its use is not warranted (and it's more expensive,more trouble than it's worth,etc.). If a cooling shaker is not available- grow the cells at room temperature.More discussions on making competent cell as well as references can be found in TIBS articles "Preparing μltra-competent E.coli" and "Better competent cells" It is also possible to transform cells straight from plate.It is convenient but you should ex pect low efficiency.See the following reference for more details (as well as more informati on on competent cells and other protocols):