Ethan Frome - Agilent Technologies
advertisement

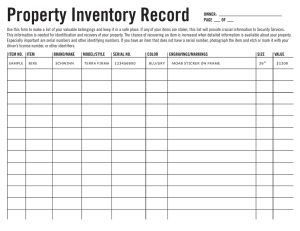
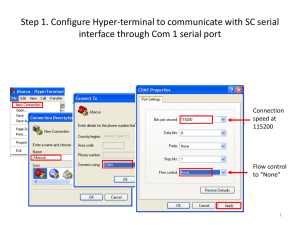
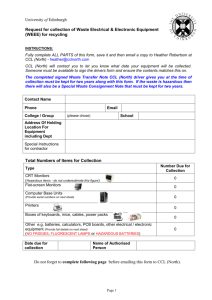
ENVIRONMENTAL HEALTH AND SAFETY FORM PLEASE FILL OUT ALL INFORMATION AND RETURN FORM WITH YOUR SHIPMENT. Please fold and insert this form into the shipping label holder in a HIGHLY VISIBLE LOCATION ON THE EXTERIOR OF EACH CONTAINER. PRODUCTS WILL NOT BE ACCEPTED WITHOUT FORM.* Please RETURN to: Agilent Technologies Deutschland GmbH c/o LGI /Willi Beetz Attn: Returns Hambruecker Landstrasse 8 68753 Waghaeusel - Wiesental Germany Phone: +49-7243-602-***/Fax: +49-7243-602-182 E-mail: returns_calce@agilent.com Product # ________________ Serial #______________________ Product #________________Serial #________________ Product # ________________Serial #______________________ Product #________________Serial #________________ Date: ________________ Returns Credit Number: ________________ Sales Order Number: ________________ Name: ____________________________________________ Company: ___________________________________________ Address: ____________________________________________________________________________________________________ City/State: _________________________________________ Country/Postal Code:__________________________________ Phone: ____________________________________________ Fax: ________________________________________________ A. Please state reason for the return: Shipment Error: ___________________________________________________________________________________________ OP Error: ________________________________________________________________________________________________ Defective: ________________________________________________________________________________________________ Wrong Labelling: __________________________________________________________________________________________ Duplicate Order: ___________________________________________________________________________________________ Customer Error: ___________________________________________________________________________________________ Warranty Replacement: _____________________________________________________________________________________ Damaged: ________________________________________________________________________________________________ By: Carrier Forwarder Customer Other ___________________________________________________ Other: ___________________________________________________________________________________________________ ======================================================================================================== B. To protect our employees from exposure to various hazards, you MUST answer the following to the best of your ability. 1. Check if unit has unit been: Powered up? Injection made? (if no on both, skip questions and sign on bottom) 2. Is there any possibility of internal or external contamination on this equipment from any of the following? YES NO Blood, body fluids ( e.g. urine, secretions), pathological specimens YES NO Infectious substances or other bioagents (e.g. proteins, enzymes, antibodies). YES NO Regulated medical wastes YES NO Radioactive isotopes are used in the area. Detail type (ECD, isotopic labels, etc.) and activity in microcuries: _____________________________________________________________________________________________________________ YES NO Chemicals or substances that are hazardous to health YES NO Biodegradable material that could become hazardous YES NO Other hazards: ________________________________________________________________________________ 3. If you circles YES to any of the above: a. Specifically describe where (on or in) the instrument/part there might be any residual contamination (for example, blood spill on the top surface)______________________________________________________________________________________________________ b. Provide details of these hazards. Include names, material safety data sheets (MSDS) and contaminants, where possible. ____________________________________________________________________________________________________________ c. Describe your method of decontamination, if performed. Attach procedure ______________________________________________ ======================================================================================================== I declare that the information is true and complete to the best of my knowledge and belief.** Authorized Signature: ________________________________ Date: ___________________________________________ ======================================================================================================== *If additional decontamination is required, you may be responsible for costs incurred to make the product safe for servicing; Shipment of equipment may fall under other transportation regulations; All applicable regulations must be followed when returning equipment. **Agilent reserves the right to reject all returns, upon inspection.