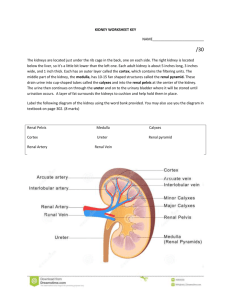
Kidneys
advertisement

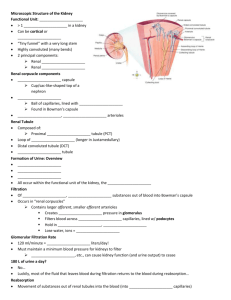
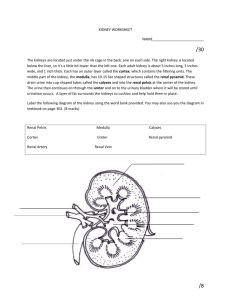
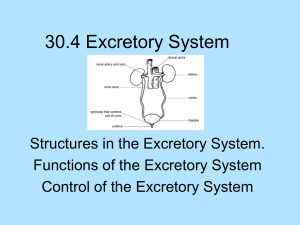
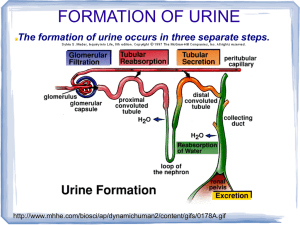
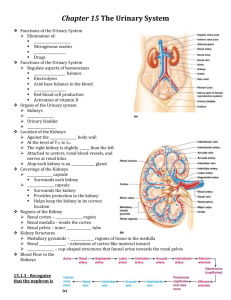
Kidneys Functions Regulation of extracellular fluid volume Removal of nitrogenous waste products, such as ammonia, urea, uric acid, and creatinine o Organisms that have access to a lot of fresh water will tend to excrete ammonia, because this byproduct takes the least amount of energy for conversion. Ammonia is very toxic to the system and requires a lot of dilution. o Urea is an intermediate which is not nearly as toxic as ammonia. o Uric acid is the least soluble of the nitrogenous products. Organisms that have little access to water usually metabolize their nitrogenous waste products as uric acid, because it can be excreted with minimal loss of water (i.e. birds). o Creatinine is the breakdown product of creatine (an important energy transfer molecule in skeletal muscle fibers transferring energy from the mitochondria to the myosin head). This is present in very stable amounts in our blood and can be used as an indicator of kidney function. If creatinine concentration in the blood is increased, it is an indication of a decline in kidney function. Removal of organic waste molecules from the blood stream Regulation of electrolytes Regulation of pH Synthesis of the hormone erythropoietin which stimulates RBC production Synthesis of Vitamin D, influencing calcium balance. Gluconeogenesis. During prolonged fasting, the kidneys synthesize glucose from amino acids and other precursors and release it into the blood. The function of the kidneys relies on having a great deal of blood flow through it in order to remove wastes and perform all of its functions. The kidney functions best at around 21-25 years of age. At age 70, the average patient has about 50% of the original kidney function. Problems related to the kidney generally affect four organs: the eye, synovial joints, kidney, and skin. It is important to remember that whatever pathology is seen in the eye is also occurring in the kidney. Anatomy The Kidney There are two kidneys (lima bean shaped, slightly reddish brown in color), one on each side of the spinal column. They are retroperitoneal, located between the peritoneum (connective tissue layer lining the abdominal cavity) and the body wall. They are around the level of the last thoracic and first lumbar vertebrae. The left kidney is usually placed slightly higher than the right. Dimensions: 11.25 cm (h) x 7.5 (w) x 2.5 (t) Capsular structures o There are three tough, thin fibroconnective tissues surrounding the kidney. o The renal capsule is the innermost layer closest to the kidney, consisting of fibrous connective tissue. It protects the kidney from infection. o The adipose capsule is the middle layer that helps in position maintenance, as well as serving as a buffer to trauma. o The renal fascia is the outermost layer consisting of dense, fibrous connective tissue. It surrounds the adrenal gland and anchors the kidneys to the surrounding structures. There are three distinct regions of the kidney o Cortex This is the outer region. It is light in color and granular in appearance. It contains the majority of the nephronal structures. o Medulla This is deep to the cortex and is reddish brown in color. It contains the cone-shaped renal pyramids with the base of the pyramid facing the cortex and the papilla (apex) facing the medulla. They are made of parallel bundles of microscopic tubules, giving it a striated appearance. The renal columns separate the pyramids. Renal papillae are at the apex of the renal pyramids and open into the internal portion, otherwise known as the renal pelvis This region contains portions of the loop of Henle. o Pelvis This is a flat, funnel shaped tube that is continuous with the ureter. Branching from the renal pelvis forms the major calyces which branch further to form the minor calyces that enclose the papillae of the pyramids. The calyces collect urine from the papillae and empty urine into the renal pelvis. Urine flows out the renal pelvis into the ureter, which is then emptied and stored in the urinary bladder. The walls of the renal pelvis contain smooth muscle, which transports urine to the bladder via peristalsis. Hilus o This is the depression on the medial aspect of the kidney, through which blood vessels enter and leave, and from which the ureter leaves. Blood Supply o Each renal artery arises from the abdominal aorta and divides into the interlobar arteries which give rise to the arcuate arteries. These arch over the base of the meduallary pyramids at the junction of the medulla and cortex and give rise to cortical radial arteries, which travel toward the surface of the cortex and supply the afferent arterioles. Each afferent arteriole leads to a glomerulus. Renal Portal System o The renal portal system contains two capillary beds in series that supply the nephron. The efferent arteriole, emerging from the glomerulus (first capillary bed), gives rise to the peritubular plexus (second capillary bed). The glomerulus (a high pressure bed) is a tuft of capillaries that receive blood through an afferent arteriole. This is the only capillary in the body drained by arterioles rather than venules. The group of peritubular capillaries (a low pressure bed) in the medulla is known as the vasa recta. They surround the nephron tubule and converge into the renal vein. Fluid filters out of the tubule and is absorbed into the peritubular capillaries via the efferent arteriole. This recaptures what was lost from the glomerulus. The glomerulus is separated from the peritubular capillary bed by the efferent arteriole which offers considerable resistance to blood flow. Therefore, the glomerular capillary bed is a high pressure bed while the peritubular capillary bed is a low pressure bed. The capillaries are low pressure to promote absorption into the blood. Note that this is not a normal pattern of blood vessels in the body. Normally blood flow is Arteriole Capillary Venule Vein Heart. Blood flow to the nephron has a different pattern. Artery Arteriole Capillary another Arteriole. The Nephron The nephron is the functional unit of the kidney. They process blood to form urine. There are approximately 1 million nephrons per kidney. Anatomy of the nephron o The glomerulus and Bowman’s (glomerular) capsule is collectively called the renal corpuscle. The Bowman’s capsule surrounds the glomerulus, with the first layer of Bowman’s capsule being simple squamous epithelium, which allows substances to cross the epithelium. The capillaries are porous, which allows solute-rich fluid to pass from the blood into the capsule. There are three layers of the glomerular capillary wall The fenestrated capillary endothelial layer keeps blood cells from blocking the glomerular filter. The basement membrane or basal lamina is an intermediate filter that keeps out only larger plasma proteins. The most selective filtration is at the diaphragms of the slit pores, formed by the podocytes. Mesangial cells are located around the glomerular capillaries that contract in response to hormones and paracrine agents to decrease the surface area available for filtration. This can be considered a modified blood vessel, therefore any disease affecting the blood vessels will affect the kidney. o Proximal tubule This 3cm tubule comes off and drains the Bowman’s capsule. This is an area of enormous amounts of metabolic movement. Simple cuboidal epithelium with prominent brush border of dense microvilli is located here to accomplish secretion and absorption. Brush border increases the surface area, actively absorbing substances from the filtrate. It is composed of both a convoluted section and straight portion that leads into the Loop of Henle Sodium is actively transported out of the proximal tubule to the peritubular blood in exchange for hydrogen ions. This creates an electrical gradient so that chloride passively follows the sodium. Water will also follow by osmosis. 65-85% of the salt and water in the original glomerular filtrate is reabsorbed back into the blood here along with 100% of amino acids, glucose, vitamins, and ketones. o Loop of Henle This portion of the nephron is composed of both descending and ascending limbs. The ascending limb leads to the distal convoluted tubule. The descending limp is simple squamous epithelium and is permeable to water. The ascending limb is divided into a thin portion made of simple squamous epithelium and a thick portion made of simple cuboidal epithelium. The purpose of the loop is to recover the remainder of water and concentrate urine. 15% of filtered water and 25% of filtered salt is returned to the vascular system. As the loop descends, solutes of the interstitial fluid of the medulla outside the tubule increase in concentration, increasing the osmotic force for water flow out of the tubule. Thus, in the descending limb, water and salt passively leave the tubule. In the ascending limb, chloride and sodium are actively pumped out of the tubule. The ascending limb is impermeable to water making the tubule fluid more dilute, therefore it is called the diluting segment. It uses a countercurrent multiplier principle to produce increasing concentration gradient. That is, the ascending and descending limbs are in close proximity to each other and “enhance” the concentration effects of each other. The vasa recta is a special U-shaped type of peritubular capillaries surrounding the loop of Henle that originates from the efferent arterioles of the juxtamedullary nephron. The capillary arrangement of blood flow through the medulla is important for maintaining the osmotic gradient set up by the loop of Henle. o Distal tubule This comes up right in between the afferent and efferent arterioles. It is also made of simple cuboidal epithelium. It lacks microvilli and secretes solutes into the filtrate. This is relatively impermeable to water. It keeps the tubular fluid dilute. This is the site of sodium reabsorption and is controlled by aldosterone. o Juxtaglomerular Apparatus This is composed of cells from the distal tubule, as well as cells of the afferent and efferent arterioles. Cells of the distal tubule make up the specialized area called the macula densa, which is a large portion of the juxtaglomerulus apparatus along with the juxtaglomerulus cells. This area secretes renin. The juxtaglomerular apparatus functions in doing most of the monitoring of the various functions of the kidney. o Collecting duct This terminates at the renal papillae marking the end. It collects urine from the multiple nephrons and runs through the medullary pyramids, fusing to form the papillary ducts at the renal pelvis. It is composed of simple cuboidal epithelium. This area is responsible for all of the “fine tuning” of the urine production. It is impermeable to water, but not salt. Water is drawn out by osmosis until the exiting urine is very concentrated. Urea us passively reabsorbed here. Permeability of the collecting tubule to water is controlled by ADH. Types of nephrons o Superficial nephron (85%) Its renal corpuscle is located a superficial area of the kidney’s cortex. The vast majority of the entire nephron is in the cortex, yet a part of the loop of Henle does dip into the medulla. o Midcortical nephron Its renal corpuscle is located in the middle of the cortex. Part of its loop of Henle dips into both the outer and inner medulla. o Juxtamedullary nephron (15%) Its renal corpuscle is located next to the medulla, in the corticomedullary border. Here, the efferent arteriole gives rise to a special type of capillary called the vasa recta. o Note that all of the renal corpuscles are located in the cortex, and all of the nephrons have loops of Henle that dip into the medulla. The closer the renal corpuscle is to the medulla, the deeper the loops of Henle dip into the medulla. Renal Physiology Glomerular Filtration (aka Ultrafiltration) This is the movement of fluid out of the glomerular capillaries and into the renal tubule. All substances that are not attached to proteins are filtered. About 20% of the cardiac output (1L) goes to the kidney per minute, which means that the entire blood supply goes through the kidney every 5 minutes. 10% of blood flow (20% of plasma flow) is filtered per minute, forming 100-125mL of filtrate per minute and 150-180L (45 gallons) per day. o When fluid enters Bowman’s capsule, it is called filtrate. Fluid leaving the renal papilla and entering the renal pelvis is called urine. The process of filtration requires that the blood move across the endothelium, basement membrane of the endothelium, basement membrane of the epithelium of the Bowman’s capsule, and through the epithelium of the capsule (through podocytes) to enter the tube. The high hydrostatic pressure (blood pressure) is the primary force driving glomerular filtration, forcing blood plasma from the glomerulus into the surrounding Bowman’s capsule. The two forces opposing this are the hydrostatic pressure inside Bowman’s capsule and the plasma oncotic pressure (colloidal osmotic pressure), resulting from the protein concentration difference across the membrane. o Net filtration pressure: Hydrostatic pressure (60mmHg) – Pressure inside Bowman’s capsule (15mmHg) – Oncotic pressure (27mmHg) = 18mmHg. This is the pressure that initiates urine formation by forcing an essentially protein-free filtrate of plasma out of the glomeruli and into Bowman’s space and then down the tubule into the renal pelvis. This small force causes a great amount of filtration. o When it comes to blood flow, also remember that the glomerular capillaries are different from other capillaries. They are larger in diameter, fenestrated, and have a pressure of 60mmHg inside, which is much higher than the regular systemic capillary pressure of 15-30mmHg due to the larger diameters and less resistance. Filtration includes both steric and charge barriers. The basement membrane, which is negatively charged, serves as the primary barrier, opposing the movement of negatively charged proteins. Generally the walls only allow for passage of small molecules (molecular weight of less than 10,000). Molecules larger than 65,000 do not get filtered. This includes large plasma proteins such as serum, albumin, globulins, and fibrinogen. Consequently the filtrate in Bowman’s corpuscle is protein-free plasma. Proteins that remain unfiltered attract water, helping resist the hydrostatic pressure in the blood. Glomerular filtration is rather nonselective, and both useful substances and waste products are presented to the tubules. The final urine differs from the glomerular filtrate in both volume and composition. Most of the valuable constituents of the filtrate (most of the salts, water, and metabolites) are returned to the blood by the tubules. Glomerular filtration rate is generally stable and will vary only a little. This is due to renal autoregulation. The juxtaglomerular apparatus stabilizes glomerular filtration by regulating the afferent and efferent arteriole. It is controlled by the effects of locally produced chemicals on the afferent arterioles. The macula densa in the juxtaglomerular apparatus detects two components of filtrate in the tubule. Regulation o Kidneys will remain a rate of urine formation over a wide range of mean arterial pressures (70-180mmHg). Due to autoregulation, when pressure falls toward 70mmHg, the afferent arterioles dilate, and when the pressure rises greater than 180mmHg, the vessels constrict. o Renal blood flow An increase in the rate of blood flow through the nephrons greatly increases the glomerular filtration rate. o Afferent Arteriolar Constriction/ Obstruction Afferent arteriolar constriction/ obstruction decreases the rate of blood flow into the glomerulus and also decreases the glomerular pressure. Both of these effects decrease the filtration rate. o Sympathetic Stimulation Decreased blood pressure causes increased sympathetic stimulation of the kidneys. Here there is vasoconstriction of the afferent arterioles, thereby decreasing the glomerular filtration rate, o o o o decreasing the urine production, increasing the blood volume, and therefore increasing the blood pressure. . Arterial pressure The glomerular filtration rate usually increases only a few percent even when the mean arterial pressure rises from its normal value to as high as 150mmHg. Efferent arteriolar constriction This increases the resistance to outflow from the glomeruli. It obviously increases the glomerular pressure, and small increases in the efferent resistance often cause a slight increase in the glomerular filtration rate. However, the blood flow decreases at the same time, and if the degree of efferent arteriolar constriction is moderate or severe, the plasma will remain in the glomerulus for a long period of time, and extra large portions of plasma will filter out. This will increase the plasma colloid osmotic pressure to excessive levels, which will cause a paradoxical decrease in the glomerular filtration rate despite the elevated glomerular pressure. Volume If the macula densa senses an increase in the volume of filtrate, it will release signaling molecules that cause vasoconstriction of the afferent arteriole so that blood flow into the glomerulus is reduced, returning the glomerular pressure and filtration rates back to normal. Electrolyte concentration As the fluids ascend the ascending loop of Henle, much of the electrolyte concentration is removed. With excess filtrate, there is not enough time for removal of electrolytes. This is why a high concentration of electrolytes reflects a high glomerular filtration rate. In response, the afferent arterioles will vasoconstrict. Low electrolyte concentration is a sign of decreased filtration rate. In response, the afferent arterioles will vasodilate, and the efferent arterioles will vasoconstrict. Tubular Reabsorption This is the movement of items such as glucose, ions, amino acids, and water out of the renal tubule, with most of it entering the peritubular capillaries. Reabsorption is the reason that the majority of the filtrate does not appear in the urine. The result is a hypertonic urine. There are two potential routes for reabsorption. First, it can go via diffusion between the tubular cells across the tight junctions. The second route is transcellular transport via active transport. The substance has to cross first the luminal membrane (separating the lumen from the cell interior) and then the basolateral membrane. An example of reabsorption via diffusion is urea. It diffuses between the cells via the concentration gradient. Most substances must use active transport due to the polarity of the cells and substances. To move water, sodium must be moved out of the filtrate. The sodium within the filtrate is moved into the tubule via facilitated diffusion and pumped out of the basolateral portion of the epithelial cell by primary active transport. The basolateral membrane has sodium pumps to pump sodium out of the cell into the interstitial tissue. This keeps the concentration of the sodium in the cell low, so that sodium moves by facilitated diffusion on the other end. 65% of filtered sodium is reabsorbed in the proximal tubule, so 65% of the water is reabsorbed. Approximately 99% of filtrate is reabsorbed, while 1% is excreted. With diuretics, 96% is reabsorbed and 4% excreted. Other substances are reabsorbed as well, such as potassium, chloride, and almost 100% of the amino acids, glucose, vitamins, ketone bodies, and molecules that are useful to the body. These are all reabsorbed in the proximal tubule, and sometimes the thick ascending loop of Henle. o Potassium and chloride reabsorption is powered by the reabsorption of sodium. Since they are moving in the same direction, this is an example of cotransport. o Generally 100% of glucose that is filtered is reabsorbed. A stark exception to this is patients with diabetes where the amount of glucose in the blood exceeds the normal limit (80-100mg/dl) due to a hormonal mechanism dysfunction. Due to the carrier mechanism and saturation kinetics, at a concentration of about 315mg/ml glucose, the glucose cannot be reabsorbed anymore and the glucose ends up in the urine. Every substance that is filtered and reabsorbed has a transport maximum, depending on the concentration that normally appears in the filtrate and how much is reabsorbed. The transport maximum value is about 375 mg/min for glucose. If there is more than 375 mg of glucose filtered per minute, then glucose will appear in the urine. This does not mean that the average patient does not have glucose in their urine. One just needs to exceed the sugar intake level in a short period of time. The difference is that those with diabetes have glucose in their urine almost all the time, because there is not enough insulin to cause the glucose to enter the cells. Due to the fact that glucose is osmotically active as it goes through the collecting duct and it carries water with it when it is being excreted, polyuria and polydipsia occurs. Therefore, more water is excreted and dehydration is more common. Reabsorption sites o The thick portion of the ascending loop of Henle is the site of sodium and chloride reabsorption, as well as potassium and H+. The thin loop reabsorbs water. o The distal tubule is the site of sodium reabsorption. o The collecting duct reabsorbs urea and water. o The proximal tubule reabsorbs sodium, potassium, HCO3-, magnesium, and chloride. Tubular Secretion This is the movement of substances such as acids, bases, and ions from the peritubular capillaries into the lumen. This occurs mainly of the distal tubule, but sometimes in the proximal tubules. Secretion increases the rate of removal of substances from the blood. Sometimes this is good (toxins), and sometimes it is bad (medicine). Important secretions o H+ and K- are moved from the tubular cells into the filtrate as sodium moves into the cell via countertransport. o Creatinine and choline o Other toxins are secreted as well, i.e. penicillin. Secretion accounts for the maintenance of the blood pH, potassium concentration in the blood, and nitrogenous waste concentration in the tubules. Renal Clearance o This is the volume of plasma from which that substance is completely cleared by the kidneys per unit time. Every substance has its own distinct clearance value. This can be further broken down into the equation CS = US * V / PS where CS is the clearance of S, US is the urine concentration of S, V is the urine volume per unit time, and PS is the plasma concentration of S. o For clinical reasons, the creatine clearance, (CCr) is commonly used to approximate the glomerular filtration rate, GFR. An increased concentration of creatinine in the urine indicates decreased kidney function. Due to the fact that there is no reabsorption and only a little amount of secretion with this molecule, it can be used as a good estimation, but it slightly overestimates the GFR. This can lead to the generalization that any substance that raises the GFR can be assumed to cause secretion. Less GFR would be due to reabsorption. Summary Part of the Nephron Glomerulus Function 1. Filtration Proximal Tubules 1. Reabsorption by active transport 2. Reabsorption by diffusion 3. Reabsorption of water by osmosis (65% salt and water) Loop of Henle: Descending 1. Reabsorption by passive diffusion 1. Reabsorption by active transport into interstitial fluid or renal medulla. A counter current multiplier is used. 1. Reabsorption by active transport 2. Facultative water reabsorption by osmosis (ADH controlled) 3. Secretion by diffusion 4. Secretion by active transport Loop of Henle: Ascending Distal and convoluted collecting tubules Substance Removed 1. Water, ions, amino acids, glucose 1. Sodium, ions, nutrients, glucose, amino acids, vitamins, ketones (100%) 2. Chloride, bicarbonate, 50% urea 1. NaCl (25%) and Water (15%) 1. NaCl (not followed by water reabsorption) since it is impermeable to water. 1. Sodium, and some ions 2. Water 3. Ammonia 4. Potassium, Hydrogen, and some drugs Water Regulation One characteristic of the kidneys is the fact there is an ability to excrete a hypertonic urine. If an istonic (300 mOsm) urine was excreted, the volume would be enormous. So to secrete this hypertonic urine, water is conserved. o Diabetes Insipidus is due to the lack of the hormone ADH. With DI, there is near isotonic urine. The most extreme form of this requires urinating 40L per day, which is equivalent to two Sparkletts water bottles. The patient would also have to ingest this much. Treatment with engineered hormones is available to help with this. When the body needs more water to maintain homeostasis, more water is retained by the kidneys. When excess water is present, more water is excreted. Mechanism to conserve water o In the nephron, the descending loop of Henle is located next to the ascending loop of Henle, allowing circulation of electrolytes through the interstitial tissue. The filtrate in the proximal tube will have the same osmotic pressure as the blood. The osmolarity goes from 300 to 1200 mOsm as the filtrate descends the descending limb. Water moves from the lumen of the tubule into the interstitial tissue, concentrating the electrolytes in the filtrate. Because the tissue has high levels of sodium and chloride, they enter the tubule, also increasing the osmolarity of the filtrate. This continues until the turnaround point. This osmolarity of the filtrate equals that of the interstitial tissue, about 1200 mOsm. The ascending loop of Henle is impermeable to water. As it goes up, sodium and chloride comes out. Going up, the electrolytes transport out of the thick ascending loop of Henle. Sodium is moved out of the limb with chloride via secondary active transport. o In the collecting duct, urea moves to the descending loop of Henle along with sodium and chloride. This drives the osmolarity to 1200 mOsm. 50% of this is due to urea and 50% due to sodium chloride. o ADH controls the permeability of the collecting duct. Function of the Vasa recta o This originates in the cortex, so the blood coming into it will have an isotonic osmolarity, but it supplies the medulla. It dips deep into the medulla, where the osmolarity of the interstitial tissue will increase from isotonic to extremely hypertonic. This is due to the fact that the capillaries are freely permeable to water and electrolytes. Therefore, solute from the hypertonic interstitium enters the descending limb of the vasa recta and water comes out, increasing the osmolarity. As the blood makes the turn and starts to ascend, water moves into the capillary and solute moves out, because the osmolarity of the blood is greater than the interstitium. Solute is being recycled during this process. By the time the blood leaves the medulla, it is slightly hypertonic. o The hypertonicity of the medullary interstitium is preserved because Blood flow occurs via the vasa recta (allowing osmotically active particles to be recycled) The medullary interstitium receives less blood flow than other body tissues. o If there was just a blood vessel going through and leaving the medulla without this countercurrent type of arrangement, all of the electrolytes would simply be washed away, and the tonicity would remain isotonic. Vasopressin (ADH) o This is a nonapeptide hormone that controls the permeability of the distal tubule and collecting duct. It is synthesized in the hypothalamus and transported to the posterior pituitary by axonal transport. The tonicity of blood is monitored by osmoreceptors in the hypothalamus. If the osmoreceptors detect an increase in osmolarity of the blood, they will cause action potentials in the neurons that synthesize ADH and release it into the capillaries of the posterior pituitary, and then travel to the kidney. It is a hormone and not a neurotransmitter, because it is actually traveling in the blood, affecting structures further away than neurotransmitters. o ADH is released in response to increased solute concentration in plasma and causes increased reabsorption of water, less excretion of water, and a more concentrated urine. Decreased levels of ADH cause the opposite. o This works, because ADH stimulates the production of cAMP which increases the number of water channels in the cell membrane of the collecting duct cells. These channels are increased in two ways. It either moves existing water channels into the membrane or makes new water channels. Vesicles are formed by the process of endocytosis. The vesicle migrates to the membrane and fuses with it. These channels are automatically inserted into the membrane. o If there is excess water (low osmolarity), ADH secretion is inhibited. Water reabsorption is minimal producing a dilute urine. Caffeine and alcohol (diuretics) both inhibit ADH secretion, and thus more dilute urine. A higher concentration of ADH would lead to maximal water reabsorption, and therefore a more concentrated urine. Normally, 99% of the water is reabsorbed. pH Regulation In general, urine tends to be acidic (pH =6). It can go as low as 4 with extreme amounts of H+. Carriers of the secreted hydrogen include phosphate (NaHPO4-), ammonia (NH3 NH4+) from ammonium picking up an extra ion, or as sulfate. Kidneys regulate blood pH by excreting hydrogen ions in the urine and by the retention and production of bicarbonate. When the blood is acidic, hydrogen is excreted and bicarbonate (a base) is reabsorbed into the blood. The opposite occurs when the blood is basic. Reabsorption of HCO3. o The bicarbonate ion (HCO3-) is one of the most important buffers in the blood. The handling of HCO3- and H+ is a much more important process than the handling of hydroxyl groups (OH-), which tend to not be much of a problem. Metabolic acidosis is a much more common issue than alkalosis, because increased pH (alkalosis) can easily be reduced by holding one’s breath for a short period of time. o Filtration leads to reabsorption, which occurs in the proximal cells. Bicarbonate cannot cross the tubule proximal wall so it is converted to carbonic acid which can cross the wall. The process is as follows: A Combination of a secreted H+ + HCO3- ion Carbonic acid (H2CO3) The carbonic acid then dissociates into H2O and CO2, which enters the tubular cells where they are converted back into carbonic acid by the enzyme carbonic anhydrase. The carbonic acid then redissociates into HCO3- and H+. HCO3- moves across the basolateral membrane by the process of facilitated diffusion. Note that the HCO3- that enters is different than the one that leaves. If there is an increase in H+ in the blood, the process of reabsorption of HCO3- takes place, because it is needed in the blood as a buffer. It does so by binding H+ forming H2O and CO2, where the CO2 can be exhaled, removing the H+. Formation of additional HCO3-. o H20 and CO2 combine within the tubular cell forming carbonic anhydrase. The carbonic anhydrase dissociates into H+, which is pumped by primary active transport into the tubular lumen. HCO3- is moved into the interstitial fluid by countertransport with Cl-. This process accomplishes pH regulation by forming new HCO3- and secreting H+. Sodium and Potassium Regulation In normal individuals, sodium excretion is increased when there is a sodium excess in the body, and the opposite occurs when there is a deficit. Most of these reflexes are initiated by various cardiovascular baroreceptors, such as the carotid sinus. Sodium and potassium balance is regulated in the distal tubule by aldosterone. Aldosterone o This is a steroid hormone (mineralocorticoid) secreted by the adrenal cortex. The secretion is controlled by the juxtaglomerular apparatus, which measures the filtrate osmolarity and volume. o Aldosterone promotes sodium retention and potassium loss from the blood by stimulating reabsorption of sodium and secretion of potassium across the wall of the distal tubules. This controls the extracellular fluid volume, therefore controlling the blood pressure regulation. A decrease in arterial pressure is detected by the juxtaglomerular apparatus, resulting in the secretion of the proteolytic enzyme, renin which cleaves proteins into smaller fragments. Renin is used to convert Angiotensinogen from the liver into Angiotensin I, which is later cleaved further to become Angiotensin II by angiotensin converting enzymes, primarily from the lungs. Angiotensinogen is always found circulating in the blood. Angiotensin II stimulates the production of aldosterone from the adrenal cortex, causing vasoconstriction to increase arterial pressure. o Renin is the rate-limiting factor. Its control includes the renal sympathetic nerves, the intrarenal baroreceptors, and the macula densa. Angiotensinogen II Pharmacological Effects o Elevation of blood pressure o Contraction of smooth muscle o Mediates release of aldosterone o Release of catecholamines from adrenal medualla and adrenergic nerves o Depresses reuptake of catecholamines by adrenergic nerves o Aldosterone primarily affects cells in the distal tubule and the cortical collecting duct to increase reabsorption of sodium. It induces the synthesis of proteins in its target cells, in this case proteins involved in sodium transport. 65% of sodium is reabsorbed in the proximal tubule, and another 5% can be reabsorbed in the distal tubule and the cortical collecting duct due to stimulation by aldosterone. If an increase in the reabsorption of sodium is required, the amount of sodium in the tubule cells is decreased by pumping more out into the interstitium. Aldosterone increases primary active transport of sodium out of the distal tubule and cortical collecting duct cells. This is done by Increasing the affinity of the sodium pump mechanism for ATP Increasing the amount of ATP available Increasing the number of pump sites By decreasing the sodium in the cell, it increases the concentration gradient across the luminal membrane. o With regards to the sodium/potassium pump, pumping potassium into the cell would cause it to diffuse out of the cell into the lumen of the tubule. At the same time sodium is being reabsorbed, potassium is secreted. This is the primary mechanism for decreasing potassium in the blood. The effect of increased potassium stimulates aldosterone secretion. This has a direct effect on the adrenal cortex. o Aldosterone controls both sodium and potassium. This is a problem in patients that take a lot of diuretics or drink large amounts of water, because it leads to aldosterone secretion above normal levels, causing decreased potassium in the blood (hypokalemia, which can cause heart attacks). Hormones working in opposition to Aldosterone o Atrial Natriuretic Hormone This is a polypeptide hormone that is made in the atria of the heart. It is released in response to increased filling of the atria (excess sodium), promoting natriuresis (sodium excretion) by decreasing sodium reabsorption in the thick ascending limb of the loop of Henle. This hormone also causes vasodilation of the afferent arteriole and vasoconstriction of the efferent, increasing the glomerular capillary pressure, which increases glomerular filtration, therefore increasing the amount of sodium filtered and excreted. o Urodilantin This is synthesized in the juxtaglomerular apparatus in response to increased sodium. Urodilantin has the same function as ANH. Calcium Regulation Both high and low levels of extracellular calcium can have varying, and even serious problems with health. The three effector sites of calcium include the bones, kidneys, and gastrointestinal tract. About 99% of total body calcium is concentrated in the bone. 60% of plasma calcium is filterable at the renal corpuscle, with most of it being reabsorbed. The rest is bound to plasma protein. Parathyroid Hormone o This is produced by the parathyroid glands embedded in the surface of the thyroid gland. Decreased plasma calcium concentration stimulates parathyroid hormone secretion, with an increased amount doing the opposite. o This hormone has four effects. It increases the resorption of bone, which results in the movement of calcium from bone into extracellular fluid. It stimulates the activation of vitamin D It increases renal tubular calcium reabsorption, thus decreasing urinary calcium excretion. It reduces the tubular reabsorption of phosphate, this raising the urinary excretion and lowering the extracellular concentration. 1,25-dihydroxyvitamin D3, aka 1,25-(OH)2D3 o This is the active form of Vitamin D. it is metabolized by addition of hydroxyl groups, first in liver cells, and then in kidney cells. Since it is made in the body, it is considered a hormone and not a vitamin. o The main action of 1,25-(OH)2D3 is to stimulate the intestine to actively absorb the calcium ingested in food. Micturition This is the process of urine removal from the bladder. The bladder is located retroperitoneally on the floor of the pelvis. In males, it lies immediately in front of the rectum. The prostrate gland surrounds the neck of the urethra. In females, it lies anterior to the vagina and uterus. Its body is a thinwalled, smooth, collapsible, muscular sac with a transitional epithelium, meaning that it goes from many layers of squamous epithelium to only a few layers as the bladders distends. It is in the shape of a three-sided pyramid with its apex pointed anteriorly. The interior of the bladder has openings for the ureters from the kidney and the urethra, which drains the bladder. The detrusor muscle is located in the middle layer of the three walls of the bladder. It consists of circular smooth muscle while both the inner and outer layers are longitudinal. There are two sphincters to the bladder- the trigone (internal sphincter) and the external sphincter. When the bladder is relaxed, the trigone collapses the opening into the urethra, closing it. The external sphincter is a circular structure of skeletal muscle surrounding the neck of the bladder. Its contraction prevents urination. The bladder normally holds about 500ml of urine, with a maximum of about 1,000ml, if necessary. By stretching the muscles, depolarization occurs and urine is strewn along the path from the kidney to the bladder. As the bladder fills (about 300-400ml), there are stretch receptors in the walls of the bladder which cause a parasympathetic reflex to occur. Action potentials go to the sacral region of spinal cord, stimulating efferent parasympathetic fibers to go back to the bladder and cause contraction. When the ditrusor contracts, pressure in the bladder increases 4060mmHg, opening the internal sphincter. The opening of the internal sphincter causes fluid to press on the external sphincter giving the sensation of having to urinate. Urination can be voluntarily prevented via descending pathways that stimulate the motor nerves to the external sphincter and simultaneously inhibit the parasympathetic nerves to the detrusor muscle. By voluntarily allowing the external sphincter to relax, urine will flow. Renal Pathology Introduction This is an extremely complex structure, with complex functions and vasculature. Because of this reason, the kidney is the target organ of a host of systemic diseases such as immune problems, diabetes, hypertension, etc. It takes about 90% loss of kidney function before signs and symptoms of pathology begin to develop. Therefore, smoldering diseases leading to loss of kidney tissue manifest very late. The average person only needs 10% to function. This is why a patient can donate a kidney and suffer no consequences. Remaining nephrons take up the slack of those lost. The amount of filtration in each nephron increases significantly, meaning that the remaining glomeruli become hyperpermeable. Infections Low blood flow (profusion) to the medulla causes difficulty fighting off infections, which can eventually lead to kidney failure. Kidney infections generally arise from bladder infections since the bladder is connected to the kidney by the ureter. These infections can be prevented by drinking a lot of fluids and by urinating when necessary, as well as taking antibiotics should an infection arise. Renal Failure Nearly any kidney disease can lead to 90% loss, leading to kidney failure. o Azotemia is the retention of nitrogenous metabolic waste products. o Uremia is the biochemical and clinical changes due to renal failure. It is due to the accumulation of nitrogenous waste products, extreme edema, acidosis, excess potassium, etc. Death due to renal failure is generally due to acidosis. Increased H+ content tends to suppress action potentials and synaptic activity. High potassium concentration can cause death as well. Kidney function is measured with BUN and creatine in a chemistry panel. o Increased BUN and creatine indicate renal failure, yet this does not show up until 90% damage. Clinical manifestations/ problems associated with failure of the system include: o Body fluids will become out of balance with failure. This usually leads to edema from salt and water retention. Edema is first seen in the eyelids, then the ankles. o Body electrolyte (K, Na, Cl, Ca, etc.) balance is out of balance with kidney failure. High potassium values seen clinically can cause heart fibrillations. o Normally, the pH is 7.35-7.45. Kidney failure leads to acidosis due to the loss of HCO3- and metabolic acid production. This causes disruption in the cellular environment, decreased enzymes, etc. resulting in possibly death. o Metabolic waste products are retained with failure, leading to azotemia and uremia. o Kidney failure leads to bone marrow suppression from “poisons” and decreased erythropoietin. This decreases red blood cells, leading to anemia. o There is a marked elevation in blood pressure with kidney failure, due to salt and water retention and increased renin. Acute Renal Failure o This occurs when the kidneys abruptly stop working entirely. The onset is very rapid (10-14 days), and full recovery is possible. o Causes An abrupt dramatic decrease in blood pressure, either because the heart stops beating or there is a huge loss of blood. This can lead to a decrease in blood flow to the kidney, causing it to be very susceptible to anoxia (loss of oxygen). A heart attack can evoke the CNS Ischemic Reflex, where blood flow to the kidney is blocked by the body so that blood may get to the brain. This is why patients that have had heart attacks could have massive kidney damage. Poisons- heavy metals (Hg, Pb) Infusion reactions where the wrong blood type is infused. Acute glomerulonephritis Other causes include renal obstructions, embolism, either extra or intrarenal, bladder rupture, vascular diseases, interstitial nephritis, pigment induction, and complications related to pregnancy. o Consequences Due to the rapid deterioration of renal function, there is an accumulation of nitrogenous waste in the blood that would normally be excreted in the urine. Edema from excess water, electrolytes, and nitrogenous waste products and other organic molecules. Can be lethal if a significant enough number of nephrons are affected. Patient should be put on immediate dialysis. Chronic Renal Failure o This is the loss of function, usually due to progressive loss in the number of nephrons. Multiple effects on many differentiated tissues are seen. o Causes Diabetes is the most common cause and can affect the kidneys by drastically increasing the rate of atherosclerotic plaque formation in the renal arteries. It also causes a thickening of the basement membrane due to an accumulation of the glycosylated end products which reduces the movement of substances across the capillary, reducing filtration. Chronic glomerulonephritis Other causes include hypertension, lupus, infections like tuberculosis and pyelonephritis, polycystic kidney disease, and obstructions. Glomerular Disease Nephrotic Syndrome o This is a noninflammatory disease of the glomerular apparatus. It is due to a host of primary kidney diseases and is often associated with systemic diseases like diabetes, autoimmune diseases, etc. It is common. o The initial event is glomerular basement membrane derangement with increased permeability to protein (primarily albumen). The increased protein decreases the serum level and the osmotic pressure leading to fluid transfer to the tissues and more salt and fluid retention via renin/angiotensin/aldosterone to maintain the intravascular volume. The decreased albumin decreases lipid transport and metabolism, therefore increasing the amount of lipids. o Signs Generalized edema, seen around the eyelids first. Decreased colloidal osmotic pressure means the ability to retain fluid in the blood is dramatically decreased, causing excessive edema. Massive proteinuria- bubbly urine! Hypoalbuminemia- decreased protein causing increased swelling due to a decreased oncotic pressure. Hyperlipidemia and hyperlipiduria Nephritic Syndrome o This is an acute inflammatory renal disease characterized by Hematuria- blood in the urine, due to inflammation Oliguria and the resultant retention of waste products (decreased urine output) Moderate Proteinuria Hypertension Mild edema usually of the eyelids and face first, which is much less than seen in the nephrotic syndrome. Azotemia o This is polygenic, mainly due to the glomerulus becoming hyperpermeable. A host of inflammatory diseases can cause the nephritic syndrome. The diseases classified with glomerulonephritis are usually immune mediated diseases, such as cross reactivity in streptococcal skin infections and lupus. o The inflammation damages the glomerular capillary wall, resulting in the red blood cells and proteins to cross the glomerular capillary to escape into the renal tubular lumen and urine, giving rise to hematuria and proteinuria. This also decreases the filtration capabilities, leading to oliguria. o Types Acute This often develops 1 to 2 weeks after a group A betahemolytic streptococcus infection of the throat, or less commonly the skin. The body forms antibodies against the proteins in the bacterium. The antigen-antibody complexes get wedged in the basement membrane between the tubule and the glomerulus, causing an inflammatory response. The proliferation of cells of Bowman’s capsule can thicken the capsule to the point where filtration cannot occur. Biopsy shows proliferation of endothelial mesangial and epithelial cells and exudation of neutrophils and monocytes. Serum complement levels are low and antistreptococcal exoenzyme titers are elevated. Most patients retain or regain normal renal function but are at risk for hypertension. Clinically over 95% of children recover. Most of the remainder develop a rapidly progressive form of the disease, and a few progress to chronic renal failure. In adults, the epidemic form has a good prognosis, but only 60% recover after the sporadic form. The remainder develop rapidly progressive disease, chronic renal failure, or delayed, but eventual resolution. Chronic This is an immunologic disorder. The mechanism is similar to acute glomerulonephritis, except that the precipitating organism is not Streptococcus. It stems from a variety of other organisms that can cause chronic conditions, not resolvable in a few weeks. The infection is not actually in the kidney, but the inflammatory response is set-up by an antigen-antibody complex. The patient remains asymptomatic for years with an insidious loss of renal function over years. The patient presents with diffuse sclerosis of glomeruli, proteinuria, hematuria, and usually hypertension. Rapid Progress An unknown absence of an immune complex is the hallmark. Pathological features include focal and segmented necrosis and epithelial cell proliferation in most glomeruli. Clinical features include fulminant renal failure, proteinuria, hematuria, and red blood cell casts. Pyelonephritis This is inflammation in the interstitium of the kidney and the tubules and not primarily in the glomeruli. Usually the renal pelvis is involved too, hence the term “pyelo.” There are a number of etiologies but the most common is bacterial infection, namely E.Coli. o Acute Pyelonephritis is almost always caused by the bacteria getting to the kidney retrograde via the bladder and ureters. It is suppurative and usually very responsive to antibiotics. Clinically there is fever, dysuria, flank/back pain, and pyuria. This is almost exclusively seen in females due to a shortened length and location of urethra, and is especially common during pregnancy. o Chronic Pyelonephritis is often a slow progressing and insidious disease which slowly destroys the kidneys. It is usually due to an ongoing, unresolved, bacterial infection. Often the only signs and symptoms are “fatigue, don’t feel well” and other vague symptoms. There is usually a little puss in the urine. There is often an undiagnosed history of “kidney infections” which leads to serious complications that warrant renal dialysis. The patient needs to be treated and followed until it is completely gone. It shows up in urine tests with white blood cells. Cystitis (Bladder Infection) This is very common, and is seen more in women. Inflammation interferes with ureter function and urine goes back up with bacteria. If it gets back to the kidney, it is then pyelonephritis. Urinary Outflow Obstruction This is a blockage of the flow of urine usually occurring in the ureters. Urolithiasis (Kidney Stone) o The formation of a calculus (stone) the size of a pin head in the collecting system. This tends to be polygenic and occurs when the patient becomes dehydrated or has other metabolic problems. Calcium, magnesium, and uric acid are most common causes. o When the stone is moving in the ureter, it tends to be very painful. Pain starts first in the flank and groin (scrotum and labia majora), then in the ureter is where it is in a band, low near the bladder in the anterior low abdomen, with sensations in the tip of the urethra. This leads to hematuria as sharp edges tear the ureter. This also leads to the smooth muscle spasm and intense waves of pain. o Treat with ultrasound to crush the stones. Tumors The primary concern is malignant tumors. The kidneys are glandular/epithelial tissue, therefore tumors here are carcinomas. o It is more common in males and more common in young/midlife, i.e. Wilm’s Tumor Renal Cell Carcinoma accounts for 90% and is 2/1 male dominated. It usually leads to hematuria and flank pain with other systemic signs of a cancer-like weight loss, fatigue, etc. Transitional Cell Carcinoma originates on the mucosal surface of the collecting system. Carcinogens are commonly cleared in the urine and in high concentration in the kidney and collecting system. o This leads to bladder cancer. This is (highly malignant) due to the transitional epithelium histology of the bladder. Treatments Dialysis Dialysis is simply the act of separating substances using a membrane. Hemodialysis o A shunt is placed in both an artery and vein. Blood will then leave the body and go through a dialyzer, consisting of a cellophane membrane that allows molecules smaller than a protein to escape. The tubing rests inside of a fluid that contains ions in concentrations similar to that of plasma. As blood goes through, an equilibrium is set up between the fluid surrounding the membrane and the blood within it. o Patients with acute renal failure may undergo this process for only days or weeks. Someone with total renal failure undergoes this process 3-4 times a week for 6-8 hours each session. A trained technician must be there the entire time, and it is a very expensive process. People on dialysis live about five years and usually die from infections. Peritonial o The patient connects themselves to a machine that pumps fluid into their abdomen where it stays there for two hours and is then pumped out. This is less expensive and can be done in home. The rate of serious infection is greatly reduced, yet it is a lot less effective. The treatment of choice for those with chronic renal failure is a kidney transplant. Diuretics These are more commonly known as water pills. They increase renal water excretion of solute and water. Most do this by decreasing renal tubular reabsorption of sodium chloride and water. The regulation of extracellular fluid volume is a very important medical maneuver for controlling blood pressure among other systemic problems. It is the first line of treatment. Diuretics are also used to treat edema, congestive heart failure, pregnancy, premenstrual tension, and to control the renal toxicity of certain other drugs. Their efficacy may be predicted from their site of action. They work depending on the ability to increase solute excretion through one of the following mechanisms o Increasing the glomerular filtration rate. o Decreasing the rate at which sodium is reabsorbed from the glomerular filtrate by the renal tubules. o Promoting the excretion of sodium by the kidney. Osmotic diuretics o Mechanism- Inhibition of reabsorption of water These are molecules that are administered and are not reabsorbed due to their large size. As they pass through the tubule, they will increase the osmolarity of the filtrate in the collecting duct, creating less of a difference between the filtrate and the interstitial tissue. This causes less of a tendency for water to move out of the filtrate at the proximal tubule, descending limb, and collecting tubule, increasing the volume of urine. o Agents Mannitol (Osmitrol) This is the most common agent and is administered intravenously. Mannitol is not metabolized, decreasing the extracellular fluid volume. The osmotic pressure of the blood is increased and then freely filtered, but not reabsorbed, increasing the osmolarity of the filtrate. This is not prescribed for chronic treatment, because it has to be infused directly into a vein. It is used in emergency situations, and the blood pressure needs to be lowered fairly quickly, i.e., acute diuresis for intracranial surgery. It has a short duration of action (2-3 hours) and safe, with very little side effects. Glycerol This is administered orally, and is very sweet. Since it is a carbohydrate, it should not be given to those with diabetes. Urea is administered intravenously. This is not as effective as mannitol mole for mole because it is reabsorbed to some extent (50%) by the renal tubules. Isosorbide (Isordil) is administered orally. o Uses Edema Glycerol and isosorbide can reduce IOP. Mannitol is used in the treatment of intracranial pressure and hypertension. o Adverse effects Initial hypertension Headache, nausea, vomiting, and expansion of extracellular fluid Mannitol can cause hyponatremia or hypernatremia (unpredictable) Carbonic Anhydrase Inhibitors o Mechanism- Inhibition of carbonic acid formation Inhibiting carbonic anhydrase reduces the reabsorption of bicarbonate in the proximal tubule. Bicarbonate is osmotically active, so it increases the osmolarity of the filtrate. These have only a mild diuretic effect since the loop of henle is able to reabsorb the excess NaCl. o Agents Acetazolamide (Diamox) This was the first tolerated oral diuretic. Methazolamide (Neptazane) Dichlorophenamide (Daranide) This is used mainly for respiratory acidosis. o Uses Prevent/treat altitude sickness Treat POAG Alkalinize the urine (increases HCO3- in urine, making the diuretic work longer) Elevated systemic bicarbonate o Adverse effects Metabolic acidosis (increased HCO3- in urine) Renal stones CNS depression Hypokalemia Thiazide diuretics (Thiazides) o These are long-acting agents whose hypotensive effects are due to decreased blood volume and arterial dilution. o Mechanism- Inhibition of chloride reabsorption in the thick ascending portion of the Loop of Henle and in the early distal tubule, resulting in 510% excretion of NaCl. o Agents Chlorothiazide (Diuril) This is a p-carboxy cogener of sulfanilamide. Hydrochlorothiazide (Hydrodiuril, Esidrix) Chlorthalidone (Hygroton) o Uses Treat edema Treat HTN and CHF Treat DI Increases water put out through the kidneys Prevents urine dilution and decreased urine output by 50% (paradoxical) o Adverse effects Weakness, paresthesia, carbohydrate intolerance. Increased plasma uric acid (gout) Potassium depletion (hypokalemia) Metabolic alkalosis- neutralize with CA Inhibitor Loop Diuretics o These are very powerful, short-acting diuretics. They are derived from two types of chemistry, sulfonamides and sulfhydryl-reactive. o Mechanism- Inhibition of sodium and chloride reabsorption in the thick ascending portion of the loop of henle. These reduce or abolish the osmotic gradient of the medulla by inhibiting chloride and sodium reabosrption. 30-40% of the sodium chloride is normally reabsorbed in the ascending loop, and there are no sites downstream capable of reabsorbing so large a load. Because of the strong potency, these can be life-saving drugs. Although these are effective, there is a “high ceiling effect.” The problem encountered with this medication is that keeping the sodium in the filtrate tends to result in potassium excretion, because retained sodium causes aldosterone secretion, increasing sodium reabsorption in the distal tubule, leading to excess secretion of potassium. So, potassium supplements must be taken. Loop diuretics decrease medullary hypertonicity, which increases the volume of the urine. o Agents Furosemide (Lasix)- lasts 6 hours Ethacrynic acid (Edecrin) Bumetanide (Bumex) o Uses Treat edema, especially in patients with low glomerular filtration rates. Treat hypertension Treat pulmonary edema o Adverse effects Increased plasma uric acid Dehydration Potassium depletion (hypokalemia) Metabolic alkalosis Sodium Channel Blockers o These are also known as Potassium Sparing Diuretics or Aldosterone Antagonists, because they do not have the effect of increasing potassium secretion. The problem with these is that they are not that powerful since only 5% of urinary NaCl is normally reabsorbed at this site, so they are given with small amounts of loop diuretics. o Mechanism- Blockade of aldosterone receptors and sodium channels in the collecting ducts. o Types Spironolactone (Aldactone) This is the only aldosterone antagonist. It takes time to develop its effect. Mechanism- promotes increased sodium and chloride excretion and reduced potassium excretion. Uses o Treat hyperaldosteronism o Counteract potassium loss induced by other diuretics o Treat edema Triamterene (Dyrenium) and Amiloride (Midamore) Probable mechanism- Inhibition of renal epithelial sodium channels Use: counteract potassium loss induced by other diuretics o Adverse effect- hyperkalemia, CI irritation, and leg cramps Summary Class CAI Loop Diuretics Thiazides K-sparing diuretics Mechanism Inhibit secretion of H+; this causes less reabsorption of sodium and bicarbonate Inhibit Na, K, 2Cl cotransport in luminal membrane Inhibit Na, Cl cotransport in luminal membrane Inhibit action of aldosterone. Also block Na channels in luminal membrane. Major Site Affected Proximal Tubules Ascending loops of Henle Distal convoluted tubule Cortical collecting ducts Uricosuric Agents Gout is associated with increased body stored of uric acid. Acute attacks involve joint inflammation caused by precipitation of uric acid crystals. These agents are used to increase the urinary excretion of uric acid and decreases serum urate levels. Mechanism o It competes with uric acid in the renal tubule for reabsorption by the weak acid carrier mechanism. At low doses, they may also compete with uric acid for secretion by the tubule and can elevate the serum uric acid concentrations. o They act primarily on the kidneys, but inhibit the secretion of other weak acids in addition to inhibiting the reabsorption of uric acid. Types o Benemid (Probenecid) Used for the treatment of hyperuricemia associated with gout and gout arthritis. o Anturane Its pharmacological activity is the potentiation of the urinary excretion of uric acid. It is useful in reducing the blood urate levels in patients with chronic gout and acute intermittent gout, and for promoting the reabsorption of tophi. Contraindication for patients with peptic ulcers and gastrointestinal inflammation. o Sulfinpyrazone o Other drugs used in gout include colchicine (inhibits inflammation of acute gouty arthritis), indomethacin, allopurinol, and aspirin.