srep04501-s1

One-Pot Synthesis of Graphene-Supported Monodisperse Pd
Nanoparticles as Catalyst for Formic Acid Electro-oxidation
Sudong Yang a,c,d†
, Jing Dong b†
, Zhaohui Yao b*
, Chengmin Shen a
, Xuezhao Shi a
,
Yuan Tian a
, Shaoxiong Lin a
, Xiaogang Zhang c* a Beijing National Laboratory for Condensed Matter Physics, Institute of Physics,
Chinese Academy of Sciences, Beijing 100190, P. R. China b School of Aerospace Tsinghua University, Beijing 100084, P. R. China c College of Material Science and Engineering, Nanjing University of Aeronautics and Astronautics, Nanjing 210016, P. R. China d Laboratory of Eco-Materials and Sustainable Technology (LEMST), Xinjiang
Technical Institute of Physics & Chemistry, Chinese Academy of Sciences, Urumqi
830011, P. R. China
†
contributed equally to this work
* To whom correspondence should be addressed: E-mail: yaozh@tsinghua.edu.cn; azhangxg@163.com
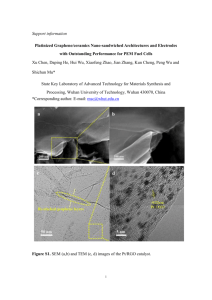
Figure S1.
XRD pattern of the (a) GO and (b) RGO.
Figure S2. TEM images of the Pd/RGO composites synthesized in typical conditions:
(a) in absence of both TOP and OAm; (b) in absence of TOP; (c) in absence of OAm;
(d) with 0.2 mL TOP and 7 ml OAm.
Figure S3. TEM images of the Pd/RGO composites synthesized in typical conditions:
(a) with 0.2 mL TOP; (b) with 0.6 mL TOP.
Figure S4. TEM images of Pd/RGO composites synthesized from (a) Pd(C
2
H
3
O
2
)
2 and (b) Na
2
PdCl
4
precursors. Other synthesis conditions are same as those for
Pd/RGO.
Figure S5. TEM images of the Pd/Vulcan XC-72 composites synthesized in typical conditions.
Figure S6 XPS spectra of Pd/RGO composite in absence of both TOP and OAm: (a)
XPS full spectrum of Pd/RGO and (b) Pd spectrum.
Figure S7. (a) TEM image of Pd NPs prepared using Pd(acac)
2
as precursor in the present of NMP solution; (b) electron diffraction pattern for the selected area.
Synthesis of Pd NPs:
NMP can be as reduced regent to facilitate complete reduction. In the redox process, the metal ions are reduced to the metallic state and nucleate to form NPs. At the same time the NMP probably are oxidized to organic compounds containing amide groups. To further confirm that, we used Pd(acac)
2 as precursor and NMP as solution to prepare Pd NPs at 200 o
C. The monodispersive Pd nanospheres with a mean diameter of 80 nm have been obtained.
19.2 mg of Pd(acac)
2 and 20 mL of NMP solution was mixed in a flask. The solution was heated to 60 ℃ and kept at this temperature for 10 min. Then it was heated up to 200 ℃ for 2h. The resulting solution was cooled to room temperature, and then 30 mL ethanol was added to yield a black precipitate which was collected by centrifuging (6000 rpm). The resulting black precipitate was obtained. The Pd NPs can be dispersed into aqueous solution.
Figure S8 TEM images of the Pd/RGO nanostructures sampled at different times during the reaction, 1-120 min.
To elucidate the shape evolution of the Pd NPs on the surface of RGO, different stages of the formation of these nanostructures have been studied and the results are presented in Figure S8. The TEM results show that the size of the Pd NPs was 3.8 nm and remained unchanged at the different stages of the reaction as time passed. As shown in Figure S8, only a few Pd NPs in random positions are formed in the first stage, most likely the discrete Pd NPs. As the Pd reduction continues, Pd atoms are added continuously, resulting in continuous growth of the area of monodisperse Pd particles, and then more and more Pd NPs begin to grow on the RGO surface until the
Pd precursor in the reaction solution is completely consumed. While the detailed mechanism remains elusive, GO is clearly critical for attaining monodisperse palladium NPs on the RGO surface. GO has two-dimensional sheets that have various oxygen functional groups ( e.g.
, hydroxyl , epoxy and carboxyl) on their basal planes and edges due to oxidation. These functional groups serve as anchor sites for Pd NPs attaching on the RGO surface (see Figure S9). When Vulcan XC-72 is in the solution instead of GO, the synthesis yields a non-uniform distribution of Pd NPs on the surface of Vulcan XC-72. The oxygen groups on the GO surface obviously have a profound impact on the monodisperse Pd nanostructure of Pd/RGO composites. In addition, some residual NMP or NMP by-product could be present at the sheet surface in the NMP system, resulting in the presence of a N XPS peak. This result could also impact the monodisperse Pd nanostructure for Pd/RGO composites.
Figure S9 Illustration of the synthesis procedure of monodisperse palladium NPs on
RGO in the NMP system.
Figure S10 (A) Cyclic voltammogram of different catalysts in 0.25 M H
2
SO
4
+ 0.25
M HCOOH solution at a scan rate of 50 mV/s; (B) Chronoamperometric curves of different catalysts in 0.25 M H
2
SO
4
+ 0.25 M HCOOH solution at 0.1V (vs.
Ag/AgCl). The catalysts in the two panels are: (a) Pd/RGO (with 0.4 mL TOP); (b)
Pd/RGO (with 0.2 mL TOP); (c) Pd/RGO (with 0.6 mL TOP); (d) Pd/XC-72.
Figure S10A shows the typical cyclic voltammograms for different catalyst-coated electrodes in 0.25 M HCOOH and 0.25 M H
2
SO
4
solution. The highest peak currents were found on Pd/RGO (with 0.4 mL TOP) electrode. It is nearly 10.5 times that on
Pd/XC-72 electrode catalysts. The Pd/RGO catalyst synthesized with 0.4 mL TOP has the best catalytic performance among all catalysts due to the monodisperse Pd NPs are formed on the surface of RGO.