Supporting information
advertisement

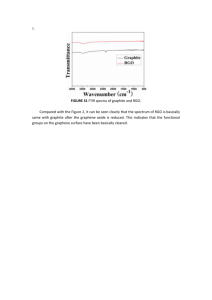
Supporting information Enhancement of H2 evolution over new ZnIn2S4/RGO/MoS2 photocatalysts under visible light Ning Ding,1 Yuzun Fan,2 Yanhong Luo,1 Dongmei Li,1a and QingboMeng1a 1 Key Laboratory for Renewable Energy, Chinese Academy of Sciences (CAS), Beijing Key Laboratory for New Energy Materials and Devices, Beijing National Laboratory for Condensed Matter Physics, Institute of Physics, CAS, Beijing 100190, 2Key China Laboratory of Bio-Inspired Smart Interfacial Science and Technology of Ministry of Education, School of Chemistry and Environment, Beihang University, Beijing 100191, China Email: dmli@iphy.ac.cn, qbmeng@iphy.ac.cn Syntheses of photocatalysts Graphene oxide (GO) was prepared by following the reference. 1 The ZnIn2S4/RGO composite catalyst was synthesized by solvothermal method. In brief, 0.136g ZnCl2 and 0.586g InCl3·4H2O were dissolved in 60mL DMF/ethylene glycol(EG) mixed solvent (V: V= 1: 1). Then, 0.3g thioacetamide (TAA) was added under vigorous stirring, which was transferred to a 100mL Teflon liner. The reaction mixture was heated at 220°C for different hours. After being cooled down to room temperature, the precipitate was filtered, washed with water and ethanol for several times, and finally dried in the oven at 70C. The ZnIn2S4/RGO composite samples were obtained by ZnIn2S4 dispersed in GO solution in 60mL DMF/EG mixed solvent, which were synthesized by solvothermal method at different reaction time. The samples were labeled as ZIS/RGO-X-Y, X represents reaction time, Y represents the percentage of weight of RGO in the sample). ZIS/RGO represents the ZnIn2S4/0.51 wt%RGO sample. Characterization The X-ray diffraction (XRD) was done by a Bruker X-ray diffractometer with Cu Kαas the radiation source. Scanning electron microscopy (SEM) images were obtained with an FEI-SEM (XL 30 S-FEG). Transmission electron microscope (TEM) image were obtained on FEI Tecnai F20 Super-twin. The thermogravimetric (TG) analysis was carried out with Netzsch STA 449F3 with temperature increases 10°C/min. FT-IR spectra were obtained on a TENSOR 27 spectrometer, BRUKER. UV–vis diffuse reflection of the samples in the range 350 ~ 850 nm was determined on UV-2550 spectrophotometer, Shimadzu. The photocatalytic reactions were carried out in a Pyrex reaction cell connected to a closed gas circulation and evacuation system. 0.1g photocatalyst sample and 0.068mg (NH4)2MoS4 solution were added into 100mL aqueous solution containing 10mL lactic acid as sacrificial reagent under stirring. Then, the system was vacuumized and exposed in 300W Xe lamp (PLS-SXE300, Trusttech) with an optical filter (λ> 420nm) to cut off ultraviolet light and a water filter to remove infrared light. The temperature of the reaction solution was maintained at 293±1.5K by a flow of cooling liquid. The evolved H2 amount was determined by an on-line gas chromatography with a thermal conductivity detector (SP6890, molecular sieve 5 A column, Ar carrier). Photocatalytic activities were compared by the average H2 evolution rate in the first 5hr. Measurement of quantum efficiency The quantum efficiency was tested with the equation I= nhv to calculate the total photo number involved. Here, I, n and v represents the light intensity, photo number and the frequency of light, respectively. Therefore, the apparent quantum efficiency (AQE) equals to the ratio of doubled H2 numbers and n. FIG. S1. FT-IR spectra of as-prepared ZIS-6h, ZIS/RGO-6h-0.05 and GO samples. FIG. S2. UV-Vis absorption spectra of as-prepared ZIS and ZIS/RGO samples FIG. S3. The stability of ZIS/RGO samples in 5h FIG. S3 shows the stability of the sample in the first 5 hours. The decreasing was considered to be the result of increasing backward reaction as the concentration of H2 increasing. To prove this, the photocatalytic system is evacuated and 5 more hours’ photocatalytic reaction was texted. The result was approximately the same as the first 5 hours, which proves that the decreasing is caused by the increasing concentration of H2. Table S1 The H2 evolution rates with different MoS2 loading amount Loading amount of MoS2 H2 evolution rate (mmol/h·g) 0.375 wt% 0.80 0.425 wt% 0.475 wt% 1.62 1.16 References 1 T. F. Yeh, F. F. Chan, C. T. Hsieh, and H. Teng, J. Phys. Chem. C. 115, 22587 (2011).