STANDARD 1 review MODULE 5 LEARNING TARGET 2c.) Observe
advertisement

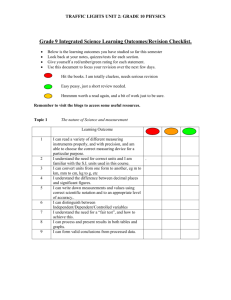
STANDARD 1 review MODULE 5 LEARNING TARGET 2c.) Observe and describe chemical reactions involving atmospheric oxygen (e.g., rust, fire, respiration, photosynthesis). VOCABULARY: Atmospheric Oxygen: The oxygen that makes up part of the air we breathe. Photosynthesis: The process of plants using sunlight to make sugar/food. Combustion: A chemical change involving oxygen and fuel producing heat and light. Oxidation: The process of one substance combining with oxygen to produce a new substance. Rusting: The chemical process of combining oxygen with iron. Respiraton: The process of combining oxygen with fuel/food to produce energy. Products: The substances that are produced due to a chemical change. Another word for new substance. Reactants: The substances that enter into a chemical change. The starting substances. LEARNING ACTIVITES SMARTNotes Differentiated Reading Activity with Quizzlettes Green Reading Resource Yellow Reading Resource Red Reading Resource Handout Handout Key ESSENTIAL QUESTIONS S1O2c: 1.) List at least 4 chemical reactions that involve atmospheric oxygen. 1.) 2.) 3.) 4.) 5.)??? S1O2c: 2.) Describe how you know if a chemical equation/reaction involves oxygen? . S1O2c: 3.) Which substances are necessary for plants to produce sugar during photosynthesis? A. water, soil, chlorophyll C. water, carbon dioxide, light B. water, minerals, cell wall D. water, roots, oxygen EXPLAIN YOUR ANSWER!!!! S1O2c: 4.) What are the reactants for photosynthesis? A. soil and chlorophyll C. water and minerals B. oxygen and carbon dioxide D. water and carbon dioxide EXPLAIN YOUR ANSWER!!!! S1O2c: 5.) What materials are required for combustion to occur? S1O2c: 6.) What do all the following chemical reactions have in common? combustion respiration photosynthesis rusting A. they all involve iron C. they all involve carbon B. they all involve carbon dioxide D. they all involve oxygen ESSENTIAL QUESTIONS KEY S1O2c: 1.) List at least 4 chemical reactions that involve atmospheric oxygen. 1.) respiration 2.) photosynthesis 3.) combustion/burning 4.) rusting 5.)??? S1O2c: 2.) Describe how you know if a chemical equation/reaction involves oxygen? the reaction will have an O2 in it. This represents atmospheric oxygen. S1O2c: 3.) Which substances are necessary for plants to produce sugar during photosynthesis? A. water, soil, chlorophyll C. water, carbon dioxide, light B. water, minerals, cell wall D. water, roots, oxygen EXPLAIN YOUR ANSWER!!!! C, these are the substances that go into the reaction. S1O2c: 4.) What are the reactants for photosynthesis? A. soil and chlorophyll C. water and minerals B. oxygen and carbon dioxide D. water and carbon dioxide EXPLAIN YOUR ANSWER!!!! D, because they are on the left side of the reaction equation S1O2c: 5.) What materials are required for combustion to occur? fuel, oxygen, ignition S1O2c: 6.) What do all the following chemical reactions have in common? combustion respiration photosynthesis rusting A. they all involve iron C. they all involve carbon B. they all involve carbon dioxide D. they all involve oxygen FORMATIVE QUIZ 5A 1. Which substances are necessary for plants to produce sugar during photosynthesis? A. water, soil, chlorophyll C. water, carbon dioxide, light B. water, minerals, cell wall D. water, roots, oxygen 2. Use the chemical equation for respiration below. What substances are needed for respiration to occur? C6H12O6 + 6O2 = A. sugar and water C. water, carbon dioxide 6CO2 + 6 H2O + Energy (heat and mechanical) B. sugar and oxygen D. water, roots, oxygen 3. Use the data table below. What is the main difference between combustion and respiration? a. the products formed c. how quickly they happen Reaction b. the fuels that are burned d. they are identical Rust Speed/Reaction Rate Measured In Hours Fire/combustion Minutes/seconds Respiration Hours Photosynthesis Hours Reactants Products Metals and water or other oxygen source Fuel (carbon compounds) Fuel (carbon compounds) Carbon dioxide, and water Rust Carbon dioxide and water Carbon dioxide and water Sugar and oxygen 4. Use the chemical equation for rusting below.What are the products of rusting? a. 4Fe + 3O2 b.) 4Fe c. 4Fe + 3O2 --> 2FeO3 d.) 2FeO3 4Fe + 3O2 --> 2FeO3 5. Why is atmospheric oxygen so important on planet Earth? a. It comes from other planets b. It does not react with many substances to form new substances c. It comes from animals d. It reacts with many substances to form new substances. 6. The process of combining oxygen with fuel/food to produce energy. a.Oxidation b. Rusting c. Photosynthesis d. Respiration 7. The oxygen that makes up part of the air we breathe. a.Oxidation b. Rusting c. Photosynthesis d. Atmospheric oxygen 8. The substances that are produced due to a chemical change. Another word for new substance. a.Oxidation b. Rusting c. products d. reactants 9. The process of plants using sunlight to make sugar/food. a.Oxidation b. Rusting c. Photosynthesis d. Respiration 10.A chemical change involving oxygen and fuel producing heat and light. a.Oxidation b. Rusting c. Combustion d. Respiration 11.The process of one substance combining with oxygen to produce a new substance. a.Oxidation b. Rusting c. Photosynthesis d. Respiration 12.The chemical process of combining oxygen with iron. a.Oxidation b. Rusting c. Photosynthesis d. Respiration 13.The substances that enter into a chemical change. The starting substances. a. reactants b. Rusting c. products d. Oxidation FORMATIVE QUIZ 5A key 1. Which substances are necessary for plants to produce sugar during photosynthesis? A. water, soil, chlorophyll C. water, carbon dioxide, light B. water, minerals, cell wall D. water, roots, oxygen 2. Use the chemical equation for respiration below. What substances are needed for respiration to occur? C6H12O6 + 6O2 = 6CO2 + 6 H2O + Energy (heat and mechanical) A. sugar and water C. water, carbon dioxide B. sugar and oxygen D. water, roots, oxygen 3. Use the data table below. What is the main difference between combustion and respiration? a. the products formed c. how quickly they happen Reaction b. the fuels that are burned d. they are identical Rust Speed/Reaction Rate Measured In Hours Fire/combustion Respiration Photosynthesis Minutes/seconds Hours Hours Reactants Products Metals and water or other oxygen source Fuel (carbon compounds) Fuel (carbon compounds) Carbon dioxide, and water Rust 4. Use the chemical equation for rusting below.What are the products of rusting? a. 4Fe + 3O2 b.) 4Fe c. 4Fe + 3O2 --> 2FeO3 d.) 2FeO3 4Fe + 3O2 --> 2FeO3 Carbon dioxide and water Carbon dioxide and water Sugar and oxygen 5. Why is atmospheric oxygen so important on planet Earth? a. It comes from other planets b. It does not react with many substances to form new substances c. It comes from animals d. It reacts with many substances to form new substances. 6. The process of combining oxygen with fuel/food to produce energy. a.Oxidation b. Rusting c. Photosynthesis d. Respiration 7. The oxygen that makes up part of the air we breathe. a.Oxidation b. Rusting c. Photosynthesis d. Atmospheric oxygen 8. The substances that are produced due to a chemical change. Another word for new substance. a.Oxidation b. Rusting c. products d. reactants 9. The process of plants using sunlight to make sugar/food. a.Oxidation b. Rusting c. Photosynthesis d. Respiration 10. A chemical change involving oxygen and fuel producing heat and light. a.Oxidation b. Rusting c. Combustion d. Respiration 11. The process of one substance combining with oxygen to produce a new substance. a.Oxidation b. Rusting c. Photosynthesis d. Respiration 12. The chemical process of combining oxygen with iron. a.Oxidation b. Rusting c. Photosynthesis d. Respiration 13. The substances that enter into a chemical change. The starting substances. a. reactants b. Rusting c. products d. Oxidation