Qualitative Analysis NO Hg
advertisement

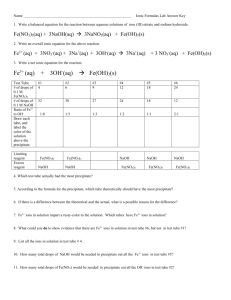
Pre-Lab Discussion Qualitative analysis is an analysis done to determine what substances are in a mixture. In this lab we will be working with four positive ions; Ag1+, Pb+2, Ni2+, and Fe3+. Our goal is to take a solution containing all of these ions, find a way to separate the solution into components each containing only one of the ions, and then perform a chemical test to confirm the presence of each of the individual ions. The method we will use to separate the ions is based on the different solubility’s of the compounds formed with these ions. The confirmations will be based on the reactions of these ions with various reagents and the appearance of specific substances when these reagents are added. The lab will consist of two parts. In the first part you will be given a mixture which contains all four ions. Following the given procedure you will separate these ions and run the tests to confirm their presence. During this part of the lab it will be important to carefully record your results so that you know what each positive test looks like. In the second part of the lab you will be given a mixture containing two to four of the ions. You will then follow the same procedure and compare your results to those in the first part of the lab. You will then be asked to determine from your results which ions were present in your unknown mixture and which ions were missing. There are three keys to being successful in this lab: 1. Keep your glassware clean. Rinse all pieces of glassware with deionized water after each use so that contamination on your glassware doesn’t give false results. 2. Label all of your filtrates and residues. If you become confused as to what residue came from where, or what filtrate is in which test tube there is no way to figure it out later. 3. Keep accurate records. Write detailed notes about what you saw in each step – this will allow you to compare your unknown mixture to the known you do the first week and consider creating an observation table to use during the lab. Objectives: Week 1: To perform and record observations of a qualitative analysis of an aqueous solution containing five known ions. Week 2: To determine the identity of unknown ions in a new sample of an aqueous solution 1 Safety: Wear goggles and an apron at all times. Tie back long hair. If any solution contacts your skin, inform your teacher and immediately rinse under cold running water. Wash hands with soap and water before leaving the lab. Exercise caution around hot plates. Materials: A. Provided in a lab bucket that includes one set of chemicals and two sets of lab glassware: Chemicals/Shared equipment: known mixture (Week 1 only), 6M HCl, K2CrO4, 6M NH3, 6M HNO3, KSCN, DMG, red litmus paper, blue litmus paper, filter paper, labeling tape Equipment (for each lab pair): 4 test tubes,1–150 mL beaker, 1 funnel , 2 stirring rods, 1 test tube brush, and 1 – 10 mL graduated cylinder B. Other Equipment (in room): Hot plate, wash bottle with deionized water, iron ring, ring stand, test tube holder, test tube rack, watch glass or Petri dish Procedure: 1. Obtain one of the carriers containing glassware and chemicals. Note each bucket contains one set of chemicals to be shared between two sets of lab partners and two sets of glassware. 2. Check to be sure your chemical bottles are at least partially full. If you have any empty bottles contact your teacher for a refill. 3. Divide the glassware between the two groups sharing the carrier. Each lab group should have a full set of the equipment list above. Obtain other equipment as listed above from room supplies. 4. If any of your glassware is not clean wash it and rinse with deionized water. There is no need to dry the glassware. 5. Place five- (5) mL of your known mixture into a clean test tube. Add two (2) mL of 6M HCl (hydrochloric acid) solution. The precipitate you will see form is a mixture of AgCl and PbCl2. Stir the mixture in the test tube with a clean stirring rod. DEFINITION: Precipitate – A solid that appears in a liquid usually as a result of an insoluble substance 2 6. Filter the solution. Collect the filtrate in your 150 mL beaker, and collect as much precipitate as possible on the filter paper. Do not rinse the test tube with water if some solid is left behind. DEFINITION: Filtrate – the liquid which comes through the filter paper when filtering a solution containing any insoluble substance. 7. Wash the precipitate on the filter paper with five (5) mL of deionized water to which you have added one drop of HCl. Collect the washings in the same beaker you used for the original filtrate. Transfer the filtrate from the beaker to a test tube and label it “Filtrate #1”. Set this aside for use in Step #12. DEFINITION: Washings – When a solid remains in a filter paper it is frequently contaminated. It is common to rinse this material with a clean liquid to remove traces of any unwanted substances. The liquid used to rinse the solid and which comes through the filter paper is referred to as the washings 8. Heat ten (10) mL of deionized water in a test tube on the hot plate. Pour this water over the precipitate in the filter paper. Collect this filtrate in a clean test tube. Call this “Filtrate #2” but there is no need to label this filtrate. Cool this test tube by running it under cold water for a couple of minutes. When cool, add one (1) mL of K2CrO4 solution. A yellow precipitate, PbCrO4 confirms the presence of the lead ion (Pb2+). This is the specific test for lead. The contents of this test tube can now be discarded. 9. Wash the precipitate on the filter paper with another twenty (20) mL of hot distilled water, again heated on a hot plate. These washings can now be discarded; however you may want to collect them in a beaker in case you poke a hole through your filter paper. If a precipitate still remains this is the positive test for silver, if no precipitate remains this is a negative test for silver. This is the specific test for silver. USE OF LITMUS PAPER: Litmus paper is used to determine if a liquid is acidic or basic. Red litmus will turn blue in a basic solution and blue litmus paper will turn red in an acidic solution. Use it as follows: Obtain a single piece of the proper type of litmus paper – blue if you are testing for an acid red if for a base. Place a single piece of paper on a paper towel. Dip a CLEAN stirring rod into the solution to be tested and touch the rod to the litmus paper, transferring a drop to the paper. The color of the wet spot will be an indication of the acidic or basic nature of the liquid tested. 10. Now take filtrate #1 which you set aside in procedure #7. Pour it back into a clean beaker. Add NH3, which is a base, until the solution which at first is acidic becomes basic (turns the red litmus paper blue). Note the litmus paper procedure above. After the solution is basic, add three (3) more mL of NH3. The dark precipitate which appears is Fe(OH)3 solid. This is NOT, however, the confirming test for iron. 11. Use a new piece of filter paper to filter the mixture you just made in procedure 10 collecting the filtrate in a test tube. Label this filtrate as Filtrate #4 and set it aside for further tests in procedure 13. 3 12. To the precipitate in the filter paper add 6M HCl drop wise until the entire solid on the filter paper dissolves. Collect the filtrate in a separate clean test tube. Add the acid until the entire solid has disappeared. Add ten (10) drops of KSCN (potassium thiocyanate) solution to the filtrate. A reddish color in the solution is the specific test for iron. The contents of this tube can now be discarded. 13. To filtrate #4 set aside in procedure 11, add ten (10) drops of DMG (dimethygloxime). A reddish –pink precipitate formed here is the specific test for nickel. 14. Scrub all of your glassware with the test tube brush and rinse it with deionized water. If one of your bottles is low, inform your teacher so it can be refilled. Please make sure you wipe your lab counters thoroughly since drops of acid and/or base may be present. Put all of the equipment/chemicals you found in the carrier BACK into the carrier. Place any other laboratory equipment back in its correct place. Week Two Repeat the procedure above using your assigned unknown instead of the known mixture. If you observe the SAME color change in a particular step then your unknown contains that ion. Mark down each positive test as you perform it. Lab Write-Up Week One 1. Write detailed observations for each step of the lab from # 5 to #13. Number your observations by step number, and write each in a complete sentence. You should accurately and specifically describe everything you observed in each step. 2. Write net ionic equations for each of the precipitated ions. (4) 3. Answer the following questions in complete sentences on a separate piece of paper: A. What was true about the nickel and iron ions which allowed you to separate them from the other two ions? B. Suppose another ion we will call X, not one of the ones we used, was in the mixture. Further, suppose X forms an insoluble chloride, is insoluble in hot water. With what ion we DID use would you find this ion? Explain both your answer and reasoning behind it in complete sentences. Week Two Create a data table with the four ions listed. Indicate next to each ion whether it was present or not present in your unknown. 4