Ch 4 Objectives / Test Review Sheet By the end of this chapter, you
advertisement

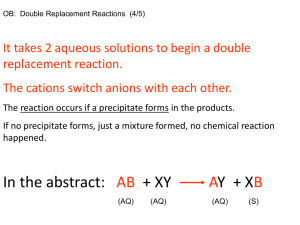
Ch 4 Objectives / Test Review Sheet By the end of this chapter, you should be able to: Identify strong, weak, and non-electrolytes. NaCl H2O CO2 NO2 LiC2H3O2 HF HC2H3O2 NH4OH Identify strong acids and bases. H2SO4 BaSO4 NaOH HBr Solve molarity problems. What is the molarity of a sodium chloride solution when 50.0 g is added to create 150. mL of solution? How many grams of calcium nitrate are needed to prepare 500. mL of a 0.200 M solution? What size volumetric flask would be needed if you wanted to make a 2 M solution of sodium hydroxide and planned to use 160 grams? Explain how to prepare molar solutions. Explain how you would prepare 100 mL of a 2.0 M NH4Cl solution. Solve stoichiometry problems involving concentration. What mass of precipitate can be produced when 125.0 mL of a 0.500 M solution of iron (III) sulfate is mixed with 150.0 mL of a 0.350 M solution of barium chloride? Predict the formation of a precipitate, given two aqueous reactants. You only need to know that ammonium, potassium, sodium, and nitrate ions are soluble! If it doesn’t contain one of these ions, assume it is the precipitate! When the following solutions are mixed together what precipitate (if any) will form? a. Na2SO4 (aq) + NH4Cl(aq) b. Fe(NO3)3 (aq) + Ca(OH)2 (aq) c. BaBr2 (aq) + Na2SO4 (aq) d. K2CO3 (aq) + Ni(NO3)3 (aq) Write molecular, complete ionic, and net ionic equations for reactions. mercury (I) nitrate and chromium (III) chloride Identify spectator ions. Identify the spectator ions in the equation above. Determine concentration of leftover ions. In a certain experiment, 150 mL of 0.500 M NaCl and 150 mL of 0.300 M AgNO3 are mixed. Calculate the amount of precipitate formed. Calculate the concentration of all the ions present after the reaction occurs. Define oxidation and reduction. Identify the oxidation numbers for elements in various compounds. Ca(NO3)2 UO22+ Mg(C2H3O2)2 HAsO2 NaBiO3 Identify element being oxidized or reduced. Cu + 2 Ag+ 2 Ag + Cu2+ SiCl4 + 2 Mg 2 MgCl2 + Si Al(OH)4- AlO2- + 2 H2O Separate oxidation/reduction into two half reactions. Balance oxidation reduction equations that occur in acidic solution. Balance oxidation reduction equations that occur in basic solution. Problems listed here as the same ones on the “More Balancing Redox Reactions” worksheet on the website. H2S + HNO3 S + NO As2O3 + NO3- H3AsO4 + N2O3 Zn + NO3- Zn2+ + NH4+ NO2 NO3- + NO2- (basic solution) MnO4- + C2O42- CO2 + Mn2+ KMnO4 + HCl MnCl2 + Cl2 + KCl Cu(NH3)42+ + S2O42- SO32- + Cu + NH3 (basic solution)