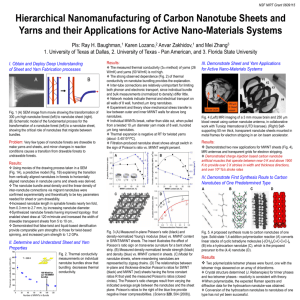
Evolution of metal catalyst during CVD synthesis of carbon nanotubes
advertisement

“Evolution of metal catalyst during CVD synthesis of carbon nanotubes” Matzinger Kuno, University of Fribourg, Department of Geosciences – Earth Sciences The revolutionary discovery of carbon nanotubes (CNT) in 1991 led to intense research activities in the domain of carbon science. The fascinating properties of these unique material has opened a great number of potential applications e.g. as electron field emitters, one-dimensional conductors, supercapacitors, reinforcing fibres or hydrogen storage. Despite these stunning technical progresses there is still much struggle in the development of a synthesis method suitable for commercial applications. A leading candidate is the chemical vapour deposition (CVD) technique. Nucleation and growth of CNTs are induced by the decomposition of carbon-containing gases (CO, CO2, C2H2, etc) over a metallic catalyst at temperatures between 600°C and 1200°. CVD is a widely used technique to generate CNTs in large quantities and much progress has been made from the point of view of the yield, the synthesis costs or the purity of the products. But the great conundrum of CVD process remains the growth mechanism. A key reaction step for nanotube growth seems to be diffusion of carbon through the metal catalyst and the most active metals are iron, cobalt and nickel but their catalytic action depends on the type of precursor, the type of substrate and of the reactive gases used. Highly controversial is the actual chemical nature of the active catalyst e.g. if it is present as metal, carbide or as mixed phase. So far few investigations of the chemical and morphological evolution of the catalyst during CVD process have been performed. This thesis focuses on the evolution of nickel-, cobalt-, chromium- and molybdenum-based catalysts under a nitrogen/acetylene and a nitrogen/acetylene/hydrogen atmosphere at 600°C and 750°C. In order to elucidate the nature of the catalyst during synthesis runs an X-ray diffractometer equipped with a heating stage and an atmosphere controlling system was used to study in-situ the evolution of metal nitrate films. Samples quenched after different pyrolysis time were investigated by SEM and TEM. The microscopic images showed that nickel, cobalt and molybdenum can act under typical nanotube synthesis conditions as catalyst for CNT nucleation and growth, but not chromium. Grain size reduction resulting from a sufficient solid volume loss during redox reactions in the catalyst precursor and the transformation of these precursors to a metallic phase are the main requirements for nanotube growth. The reaction sequences observed during the reduction of the precursor are put in relation with the nucleation and growth of nanotubes. Diffusion of carbon through the metal particle, indicated by an increase of metal cell parameters identified in diffractograms as peak shifts, was observed whenever carbon nanotubes were generated. In the nickel and cobalt system no carbide phases were detected. In contrast to the iron system, where the break-down of metastable carbides act as a second boost of nanotube formation, the appearance of carbides in the molybdenum system after 20 minutes stops further carbon nanotube growth. In any case hydrogen pre-treatment promotes nanotube growth. Jury: Prof. Dr. Bernard Grobéty (University of Fribourg, Department of Geosciences – Earth Sciences), Prof. Dr. Andreas Strasser (University of Fribourg, Department of Geosciences – Earth Sciences), Prof. Dr. Andreas Züttel (University of Fribourg, Department of Physics), Dr. Andri Vital (EMPA Dübendorf, Laboratory for High Performance Ceramics)











![Description This tool runs the model described in Ref. [1] below. It](http://s3.studylib.net/store/data/007555824_1-2f0124cd3aa95426766ad7b8bcd713b0-300x300.png)